Will Trump Give ‘Right to Try’ a Boost?
As election returns came in on November 8, Seth Bombet was as despondent as most everyone he knew. The lifelong liberal found Donald Trump mendacious, vulgar and ignorant in just about every way. This was the first — and possibly the last — presidential election Bombet would vote in, and he was distressed that this man had won over enough Americans to snatch the White House.
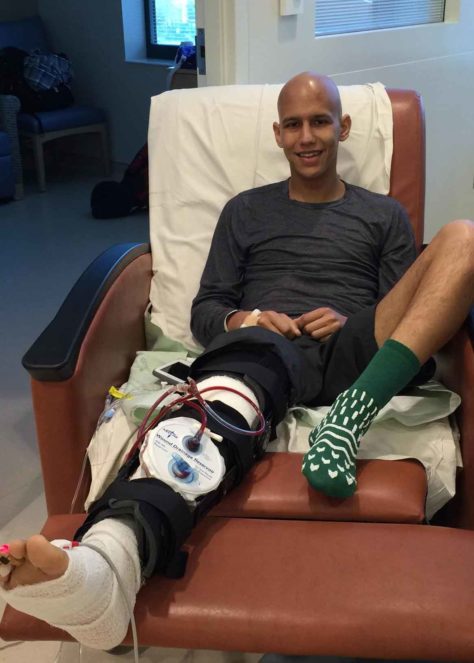
Bombet following a surgery to remove his tumor.
Visual: Charles Bombet
Yet amid his despair, the 23-year-old from Baton Rouge, Louisiana, saw one unexpected cause for optimism: he thought Trump could save his life. Bombet was a senior at Louisiana State University weighing which medical school offer to accept when, in late December 2015, he started experiencing dull leg pain. Bombet figured he had injured himself while running, but the pain intensified and soon led to a diagnosis — osteosarcoma, a bone cancer that would eventually spread to his lungs. The year became a blur of agonizing rounds of chemotherapy and surgeries that his cancer shrugged off, continuing its stubborn attack unabated.
By the time Bombet went to his local polling place on Election Day, he was bracing for the possibility of another operation — and his family was trying to figure out how to obtain a promising but unapproved medication called mifamurtide, or MTP. The drug had helped cure other osteosarcoma patients, and is part of a standard treatment in several other first-world nations, including the United Kingdom and Israel.
Bombet loathed Trump’s indiscriminate attacks on regulations and refusals to accept basic scientific premises around topics like climate change. But if Trump’s Food and Drug Administration dismantled the regulations preventing Bombet from getting MTP, then the Republican’s victory might have an upside.
“I told my sister, ‘You know, if there is one good thing that could come from this, it’s that this administration could open up more options for people who are in situations like me,” Bombet recalled in late April, from a room at St. Jude’s Children’s Research Hospital in Memphis. “Of course, [Trump] is also lifting restrictions on polluters, too, and I’m from a city by the Mississippi River where there’s all these chemical plants. It’s possible that there would be a lot less people in my situation if there were less chemical plants dumping things into the Mississippi River, too.”
Bombet’s hesitant optimism about this one aspect of the Trump Era mirrors that of thousands of patients and their supporters who believe the FDA is blocking people with chronic or fatal diseases from treatments that could help and maybe even cure them. The president himself addressed the matter in a February 28 speech to Congress: “Our slow and burdensome approval process at the Food and Drug Administration keeps too many advances … from reaching those in need. If we slash the restraints, not just at the FDA but across our government, then we will be blessed with far more miracles.”
The movement to change drug approval is known as Right to Try, and measures bearing that name have already passed overwhelmingly in 37 states. Vice President Mike Pence — who, as governor of Indiana, was an early supporter — hosted activists and patients at the White House in February to reiterate support for a national version. And Trump’s FDA Commissioner Scott Gottlieb, both a physician and a cancer survivor, has vowed to work with Congress on any RTT legislation.
Typical state-level RTT laws aim to grant terminally ill patients and their doctors the ability to work with drug companies — without needing FDA approval — to access medications that have passed the first clinical trial phase for safety. It’s unclear thus far whether these laws have had any significant, aggregate impact on the lives of patients. But RTT supporters, keenly aware of the political popularity of such measures, see them as stepping stones to broader changes in regulation, particularly with Trump in power. Many seek not just a federal RTT statute, but also automatic availability of drugs approved in other countries and access to unapproved medication for the chronically ill.
“They are building blocks on each other,” said Starlee Coleman, spokeswoman for the Goldwater Institute, a libertarian think-tank that is drafting and lobbying for RTT legislation. “They go together for us. And, yes, we are very optimistic about this White House.”
RTT opponents are as fearful of Trump as proponents are encouraged. They believe allowing access to unapproved medication can be dangerous and unethical — and also popular. That makes it an easy political calculation for Trump; here the president can push deregulation that benefits not massive corporations but distraught families and the gravely ill, says Dr. Alison Bateman-House, chair of the New York University School of Medicine Working Group on Compassionate Use and Pre-Approval Access.
“I’m very concerned right now,” Bateman-House says. “The people pushing these policies are sympathetic people. It is very hard for elected officials to find some coherent narrative to explain why they are not willing to vote the way someone who has a very compelling, tragic story would be lobbying them to vote. We sort of joke that Right to Try is like Mom and apple pie — you go against it at your political risk.”
But, she adds, “the people who are pushing these policies are either knowingly or unknowingly pushing policies that aren’t really going to help patients.”

The origins of Right to Try trace to the 1980s at the height of the HIV/AIDS epidemic, when protesters painted FDA regulators as heartless bureaucrats who stalled on promising treatments while gay men and drug addicts died. That activism led to reforms that significantly hastened the pace of drug approvals — and emboldened other groups to push policy changes at the FDA. A variety of causes célèbres followed — cases that usually involved dying children and their grief-stricken parents, who lashed out in the media about the governmental obstacle pushing possible cures beyond their reach.
But it wasn’t until the advent of social media that groups representing a diverse array of diseases coalesced into a potent movement by organizing via Facebook groups and other online meeting spaces. In 2009, in response to growing pressure, the FDA broadened its expanded access (or compassionate use) program, which allows physicians to submit requests for patients to receive “investigational drugs,” including medication not yet in clinical trials. While the arrangement leaves the initial request with the physician, it’s up to the drug manufacturers to decide whether or not they’re willing to provide the drug, and up to the FDA to give permission and dictate dosage.
To many RTT proponents, this isn’t good enough — as highlighted by the groundbreaking and highly publicized cases of two boys, Josh Hardy and Diego Morris, who both battled cancer.
In 2014, when Hardy was 7, he was close to death from what, for most people, would have been a simple cold. The virus that caused it — adenovirus — can be life-threatening when the immune system is compromised, as Hardy’s was thanks to a bone marrow transplant to treat his cancer. His family learned of the antiviral medication brincidofovir that was showing great promise in helping immune-suppressed patients combat adenovirus in the first Phase I clinical trials, which determine basic safety in humans, and Phase II, which are the first tests for efficacy. The FDA approved the use under its expanded access program, but the drug’s manufacturer, Chimerix, declined to provide it. Similar requests, the firm said, had distracted it from finishing the final trials and bringing the medication to market.
Hardy’s mother posted her frustration on Facebook, it went viral, and outlets like CNN produced scathing headlines such as: “Company denies drug to dying child.” Eventually Chimerix relented. Hardy recovered from the infection, which was seen as a huge victory, but he later succumbed to the cancer in 2016. In the meantime, brincidofovir failed the FDA’s Phase III trial — which involves largescale testing to compare a drug’s safety and efficacy to a placebo or existing treatment — in December 2015, although Chimerix continues to push a form of the drug through the approval process.
The case of Morris, now 16, was similarly high-profile. In 2012, when Morris was 11, he was diagnosed with osteosarcoma, a rare pediatric cancer that hits about 1,000 people in the United States each year (including Seth Bombet). Morris’ family learned from some physician friends about MTP, which is used after surgery in conjunction with chemotherapy to prevent cancer from returning. But, his parents said, at the time the drug had already been through the FDA approval process and was denied for use in osteosarcoma. (Sandy Walsh, an FDA spokeswoman, was unable to comment on MTP specifically, but clarified that it is possible for a drug that has been denied approval for sale and marketing in the U.S. to be provided under expanded access.)
In any case, the Morris family didn’t have time to waste. MTP had already been approved for use in osteosarcoma in Europe, so Morris, along with his brothers and mother, moved from Phoenix, Arizona to London for nine months of MTP treatment. He beat his cancer and became the face of RTT efforts, serving as an honorary chair of the 2014 campaign to pass an Arizona referendum that garnered nearly 80 percent of the vote and led to the enactment of a RTT law. Last year, Morris testified in favor of RTT before the U.S. Senate.
Diego Morris shared his story about beating cancer at a Senate hearing in February 2016.
Arizona was the fifth state to pass a RTT law. By May 2017 — just three years after the first such measure was approved in Colorado — 37 states had passed RTT laws. Today, bills have been introduced in the last 13 states as well as in both houses of the U.S. Congress. At least half of the RTT state laws passed both chambers of the state government unanimously — an astonishing feat of bipartisanship in the modern political climate.
That populist juggernaut masks an important truth: Nobody knows whether RTT laws have actually delivered unavailable medicine to any patient. The Goldwater Institute — despite its intensive lobbying and research on the matter — doesn’t have any data and could not point even to one anecdote. Indeed, even supporters question the laws’ practical or legal value without a federal component — be that an act of Congress protecting pharmaceutical companies from liability if an unapproved drug does harm, or a Department of Justice policy statement promising not to prosecute doctors, hospitals or drug firms that administered medications without the FDA’s approval. (Proponents point to other areas where the DOJ has chosen not to litigate against state measures that run contrary to federal law, such as the Right to Die, marijuana legalization, and online gambling.)
“It’s unclear whether the FDA will be supportive of these state acts or whether they’re going to push against them,” said Michael Smith, vice president of the Gastroparesis Patient Association for Cures and Treatments, or G-PACT. Until the federal authorities explicitly state whether they will prosecute doctors who dispense medicine that isn’t approved by the FDA, Smith says, the value of the RTT laws that have passed will remain in question.
While RTT opponents also insist that they aim to protect people’s health, they see the FDA’s role very differently. Bateman-House, the NYU medical ethics professor, is one of several health policy experts who want the FDA to remain central to all drug approvals. For starters, she says, FDA scientists are in the best position to judge the drugs because they have real-time access to the most information about medication and clinical trial data. Then there’s the matter of speed: despite Trump’s rallying cries — that the FDA is a lumbering, inflexible behemoth — these aren’t borne out by the facts. Bateman-House points to an April 2017 New England Journal of Medicine essay asserting the FDA drug approval process is more than three months faster than the European Medicines Agency. Similarly, complaints that the FDA’s expanded access program is too slow also frustrate Bateman-House. The agency’s own data shows it usually processes emergency requests within 24 hours, while non-emergency requests take an average of four days, she says.
Bateman-House explains the main “sticking points” as finding a physician who is willing to use an investigational drug, figuring out what’s in the pipeline and who distributes it, and then finding out if the company is willing to provide the medication under expanded access. The passage of the 21st Century Cures Act last year has made this last step easier, she explains, by requiring that as soon as a drug has entered Phase II clinical trials, the manufacturer must explicitly state whether it will be available under expanded access.
“It is definitely not helpful,” Bateman-House says of the deregulation pushed for by Right to Try advocates, “because you have to go through this whole process and everything comes to a full stop if the company says no. And a proposal that focuses on cutting out the FDA doesn’t understand that sort of trajectory. It’s focusing on the wrong issue.”
RTT laws still do not force drug companies to participate, as doing so could chill pharmaceutical development. Many are startups running on venture capital and face certain bankruptcy without FDA approval. Providing medication for compassionate use can be expensive to firms that aren’t scaled up for production beyond the quantity needed for clinical trials, and a catastrophic result can ruin the drug’s prospects by negatively skewing the data on the drug’s efficacy.
Social networks and viral posts from frustrated parents carry a lot of power. But if a drug is approved for compassionate use and it doesn’t work — or worse, it turns out to be toxic — what happens when “an outraged family discusses that drug” on Facebook and Twitter, asked Musa Mayer of the Metastatic Breast Cancer Alliance, at a New York Academy of Sciences conference in 2015. “What happens if that drug could have benefited other people? What happens to the stock of the company developing it? There are unforeseen consequences of unfettered access.”
Right to Try supporters have some answers. First, Smith says, while the outcome in terminally ill patients receiving a medication outside of clinical trials should still be reported to the FDA, it shouldn’t be held against pharmaceutical companies in any way.
More importantly, he says, the FDA shouldn’t take part in approving investigational medication for compassionate use. Instead, the decision should be left to the doctor, the drug company and the board that oversees medical practices and makes complex treatment decisions at the clinic or hospital where the drug would be administered, he says, because specialists at well-regarded medical centers are better positioned to understand the disease and the potential patients.
“It is not clear that you necessarily have all of the right people at FDA reviewing the need for these medications in these kinds of patients,” Smith says. “If you leave the current system as is, you have to hire more experts at the FDA. In our cases, some of those people must be gastroenterologists and some better be doctors who can understand the comparative risks of gut paralysis and motility.”
Challenges to the FDA’s role in approval may come from within as part of Gottlieb’s potential reforms, although he was tight-lipped about specifics in the run-up to his Senate confirmation. But this Congress looks likely to pass a federal RTT measure given its broad bipartisan support.
Advocates like Smith and Coleman are also supportive of another RTT proposal, the RESULT Act — or the Reciprocity Ensures Streamlined Use of Lifesaving Treatments — which would force the FDA to fast-track its review and approval of medications and devices approved in other countries.
“A lot of times these issues are very complex, but from a common-sense point of view, it’s hard for me to imagine that Canada, which is a pretty similar country to ours, would approve something that’s not safe,” Dr. Thomas Abell, a gastroenterologist at University of Louisville Hospital, says. Abell has long been interested in getting the FDA to approve domperidone, a drug shown to be effective in helping people with gastroparesis digest food but is only available in dire cases through the compassionate use program. “The people I see have pretty much been failed by everything else. They need this.”

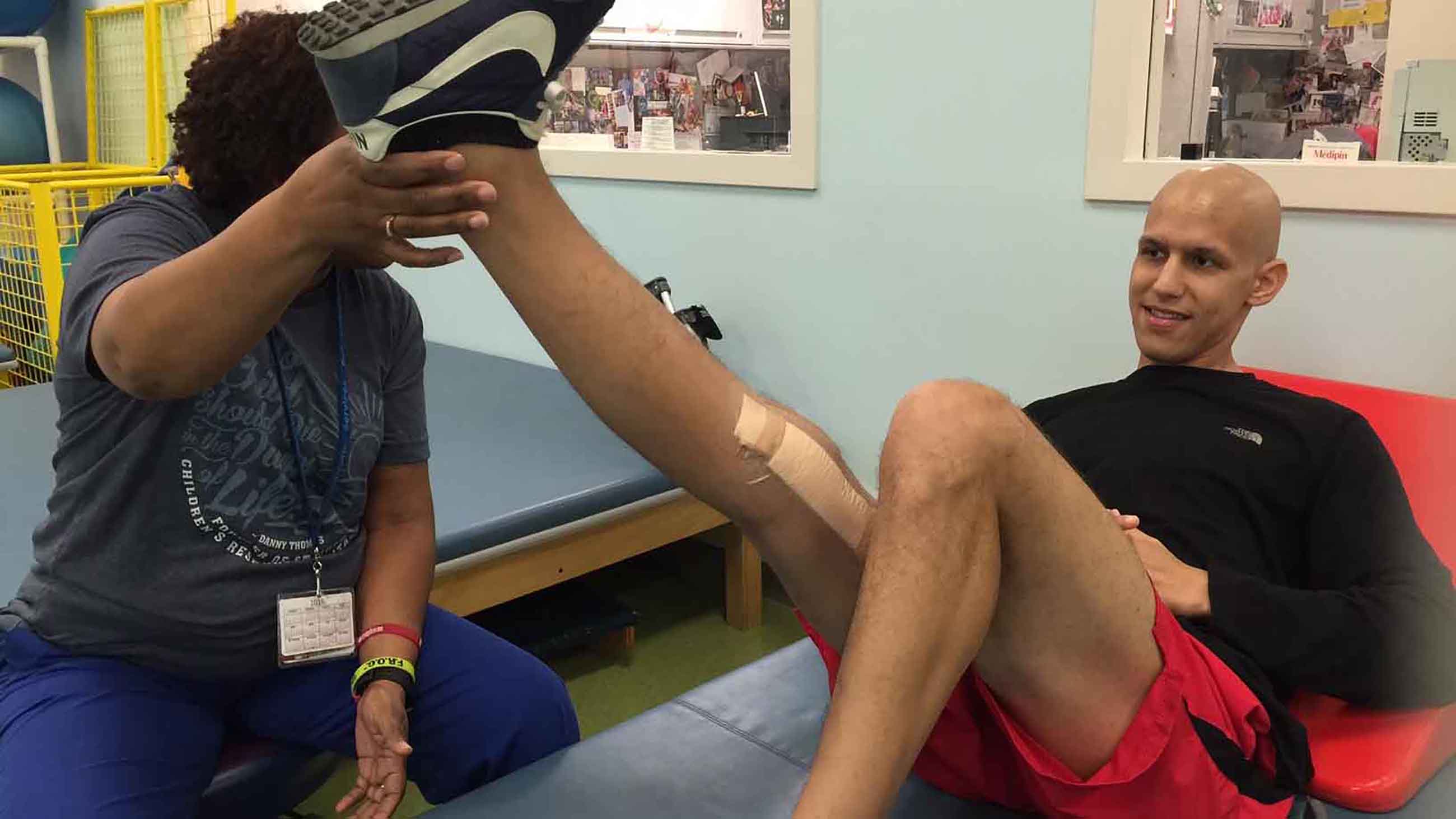
By last fall, Seth Bombet and his family were considering his other medical options. Bombet’s father, Charles, had heard about MTP, including during the February 2016 U.S. Senate testimony by Diego Morris, the kid with the same type of cancer whose family moved to London for treatment years earlier.
“My dad asked the doctor one last question,” Morris told a rapt Senate Committee on Homeland Security and Governmental Affairs. “He asked whether if, God forbid, the doctor’s child or grandchild had osteosarcoma, would he take them out of the country in order to get MTP? He responded that he would indeed travel for MTP.”
The Bombets considered a move abroad. “If I wouldn’t have gotten access to the drug at St. Jude’s, then we were talking to relatives in France, we were looking at moving to Israel,” Seth Bombet says. “My family was prepared to go halfway across the world if we had to.”
Fortunately, that wasn’t necessary. Although the Trump Administration’s take on RTT may change the fate for many patients hoping to try pre-approved drugs, that wasn’t necessary for Bombet. Late last year, the FDA approved his oncologist’s request to access MTP under expanded access. In early February, Bombet started receiving infusions of the drug twice a week at St. Jude’s in Memphis.
Bombet was supposed to remain on MTP for 36 weeks, but the treatment ended in late April after just 10. A CT scan showed that the drug was not only ineffective, but that the cancer in his lungs had grown into an inoperable tumor. On May 2, his disheartened parents brought him home to Baton Rouge to assess their dwindling options. Ultimately, they opted to head to the Cleveland Clinic in Ohio for another novel treatment — this time a multi-prong attack with chemotherapy, stereotactic radiation and infusions of the radioactive isotope radium 223.
In early May during a FaceTime call, Bombet appeared relaxed and energetic, his hair growing in thick for the first time in almost a year. He was about to travel around Canada and Europe and planned to start medical school this fall at LSU in New Orleans.
Even with the disappointing outcome, was he pleased he got the try MTP?
“Yes, absolutely,” he said with a dimpled smile before turning serious. “There aren’t a lot of options out there for a disease like osteosarcoma. So the fact that there’s something out there that is standard of care all over the world that wasn’t going to be an option to me when they weren’t offering anything else with promise — that wasn’t right.
“It’s hard to justify not being able to take a drug that could save me,” he added. “It’s hard to look past that.”
CORRECTION: An earlier version of this story referenced the life expectancy for osteosarcoma patients who undergo a treatment involving the radioactive isotope radium 223. That data actually referred to treatment for a different type of cancer. Ra-223 is still in clinical trials for inoperable osteosarcoma, so no current data on survival rates is available.
Steve Friess is a former technology and politics senior writer for Politico whose freelance work appears regularly in Time, BuzzFeed News, New York Magazine, and Al Jazeera America, among other publications. He is based in Ann Arbor, Michigan.