For a Pivotal Vaccine: Trial, Error, and Two Young Lives
The child falls sick shortly after Christmas. At first he has a cough and a runny nose. Soon his breathing grows labored. On the afternoon of Dec. 30, 1966, he arrives at Children’s Hospital in Washington, D.C. A clinical report describes him as a “well-developed, well-nourished Negro boy in acute respiratory distress.” He is 14 months old.
That evening, another child arrives at the same hospital: a previously healthy Black boy, aged 16 months, running a fever and struggling to breathe. His small rib cage flexes and caves as he sucks in air.
Hospital staff give the children oxygen, aspirin, and penicillin. But when New Year’s Eve arrives, both are in grave condition. The 16-month-old child passes away shortly after 2:30 a.m. on New Year’s Day — “somewhat suddenly and unexpectedly,” according to his autopsy report. The 14-month-old dies the next day, Jan. 2, in the late morning.
In the days that follow, senior officials at the hospital and the National Institutes of Health exchange urgent messages about the two boys’ mysterious deaths, as well as several other anomalous cases of sick children. Those cases will soon reshape an entire federal research program. Top scientists will devote portions of their careers to studying what, precisely, happened that winter.
In time, the boys’ deaths provide valuable information toward developing working vaccines for respiratory syncytial virus, or RSV, which kills thousands of older adults and sends tens of thousands of children in the United States to the hospital each year. Over five decades, scientists publish dozens of papers drawing on clinical data of the boys who died, and studying tissue samples from their autopsies.
Members of their families have no idea.
The RSV research program reached a major milestone in 2023, with the arrival of RSV vaccines. Since May, the Food and Drug Administration has approved two vaccines for use in older adults, as well as an antibody shot that helps fend off severe RSV in babies and toddlers. In August, regulators also approved giving one of those vaccines to pregnant women, to offer some protection to newborns. All evidence indicates that the shots have the potential to save thousands of lives annually, and to keep tens of thousands of small children out of the hospital.
What has been lost amid these medical milestones are the stories of the dozens of children who suffered severe bouts of illness in the early days of RSV research, after receiving an experimental vaccine that, rather than offering protection, worsened symptoms during an initial RSV infection.
“Especially if these RSV vaccines can be successful,” said vaccine scientist Barney Graham, “I would like to see some of these companies, and maybe the government, go back and do some repair work.”
Today’s vaccines are a far cry from the experimental shot that debuted in the 1960s: They’ve been rigorously tested in animals, and then vetted in phased human trials designed to catch all but the most elusive side effects. But troubling questions linger about the clinical trials that helped launch the RSV vaccine research program. The first, and youngest, children to get the vaccine were mostly Black and poor. Some were enrolled in foster care. Clinical research was largely unregulated at the time, and archival documents housed at the NIH suggest that parents did not give informed consent — or in some cases, any consent at all — for their children to receive the largely untested shot.
After it became clear the experiment had failed, researchers downplayed the risks in their communication with some families. Relatives of the two boys who died, who were identified as part of an expansive Undark investigation into early RSV vaccine research, suggest they received limited, if any, information about what had transpired. And, as the bodies of their children became objects of international scientific attention, family members were seemingly never informed of the research.
Set against the rollout of the new RSV vaccinations, the stories of these children and their families raise potent questions about the march of science and its loosely regulated past. How should researchers, doctors, and patients grapple with the legacy of those harmed by the scientific errors and ethical lapses of previous generations? And what is owed to those who suffered — or even lost their lives — in pursuit of a lifesaving vaccine?
Robert Chanock was 32 years old, married, with two young sons when he arrived at the National Institutes of Health. It was 1957 — a golden era for virus research — and Chanock’s job was to study RSV and other viruses, and to develop vaccines against them.

Robert Chanock spearheaded early research into RSV and the hunt for an effective vaccine.
Visual: Office of NIH History
It was precise, difficult work, but Chanock was well-suited for the task. In interviews, people who knew Chanock described him as brilliant, quirky, and almost preternaturally focused. Over his years at NIH, he settled into an austere routine, waking up each morning around 3:30 or 4:00 to begin work. Colleagues remember him as warm — he gave most everyone nicknames — but exacting, marking up drafts of papers with liberal strokes of a red pen. He swam 1 mile every day, and he harbored a passion for classical music, amassing a library of thousands of recordings. The scientist was in bed most evenings by 6:30 or 7:00.
He was “an obsessive character,” Chanock’s son Stephen recalled in an interview.
Chanock had grown interested in virology as a pediatrician-in-training in the late 1940s. At the time, doctors knew that viruses caused many common illnesses. But they were left to label diseases in vague terms — that’s viral croup, or that’s viral pneumonia — with little understanding of which virus was causing which illness. A series of breakthroughs, however, made it much easier to grow viruses in the lab and discover the cause of common illnesses, and Chanock became adept at doing precisely that kind of detective work. In 1956, he and colleagues at Johns Hopkins University were the first people to identify RSV in humans, using throat swabs taken from two infants in Baltimore.
The move to the NIH married Chanock’s talents with ballooning federal support for scientific research. The NIH was a small agency before World War II, with an annual budget under $500,000. But by 1957, when Chanock arrived at the agency’s large campus in the leafy Washington suburb of Bethesda, Maryland, the agency had a budget approaching $180 million. Chanock thrived there. “Never in the history of infectious disease research has one person developed so much definitive information about the causes of so much human disease in so short a period of time,” a colleague later wrote about Chanock’s early years at NIH.
The son of a Chicago factory owner, Chanock had little need for money. He devoted himself with monastic discipline to the study of infectious disease. In the family home in Bethesda, his son recalled, Chanock was responsible for just a single piece of decoration. It was a poster, hanging in the kitchen, featuring a brief quote: “There’s no greater good than the public good.”
In September 1960, in Slidell, Louisiana, Emily Lucille Jones marries Joseph Manuel Hambrick. They are high school sweethearts, still in their teens. Their first child, a son, is born in February 1961. They name him Joseph Jr. Just 10 months later, Emily gives birth to a daughter, Sharlette, at Charity Hospital in New Orleans, across Lake Pontchartrain.
Joseph has a job at a tavern and general store in Slidell, while Emily takes care of the kids. A third child, Frederick Dwight, who goes by his middle name, comes along in 1964. Soon after — the exact date is unclear — the family moves from Louisiana to Washington, D.C., where Joseph had lived for a period in his childhood, and where he has connections through his former stepfather.
The nation’s capital is undergoing a period of upheaval. White people are streaming out of the city, settling into suburbs in Maryland and Virginia where Black people are restricted from buying homes. At the same time, thousands of Black families are arriving in Washington. Many of them, like the Hambricks, are migrants from the rural South, where good jobs are scarce and segregation remains enshrined in the law.
Although segregation has officially ended in Washington, the city remains starkly divided. Many of the new arrivals, who have little formal education, struggle to find stable housing and work.
Joseph does find a job, as an orderly at the research hospital of the National Institutes of Health.
In 1961, Janet King, a 16-year-old high school student in Washington, D.C., gives birth to a son, whom she names Darius. The baby comes home to the rowhouse on Willard Street where Janet has lived her entire life, and which she shares with her mother and her younger sister. Her mother sublets a room in the family’s second-floor unit to help cover the bills.
Janet finishes high school — Darius attends her graduation — and she eventually takes a job at the Department of Commerce as an administrative assistant. She’s raising Darius as a single mother, and money is tight.
The house on Willard Street is just a block off U Street, which is known as Black Broadway. It’s a major thoroughfare, a hub of commerce and nightlife in the District. But Willard Street is quiet — just one block total. “It was very neighborly,” Darius would later recall. “We never locked our door.”
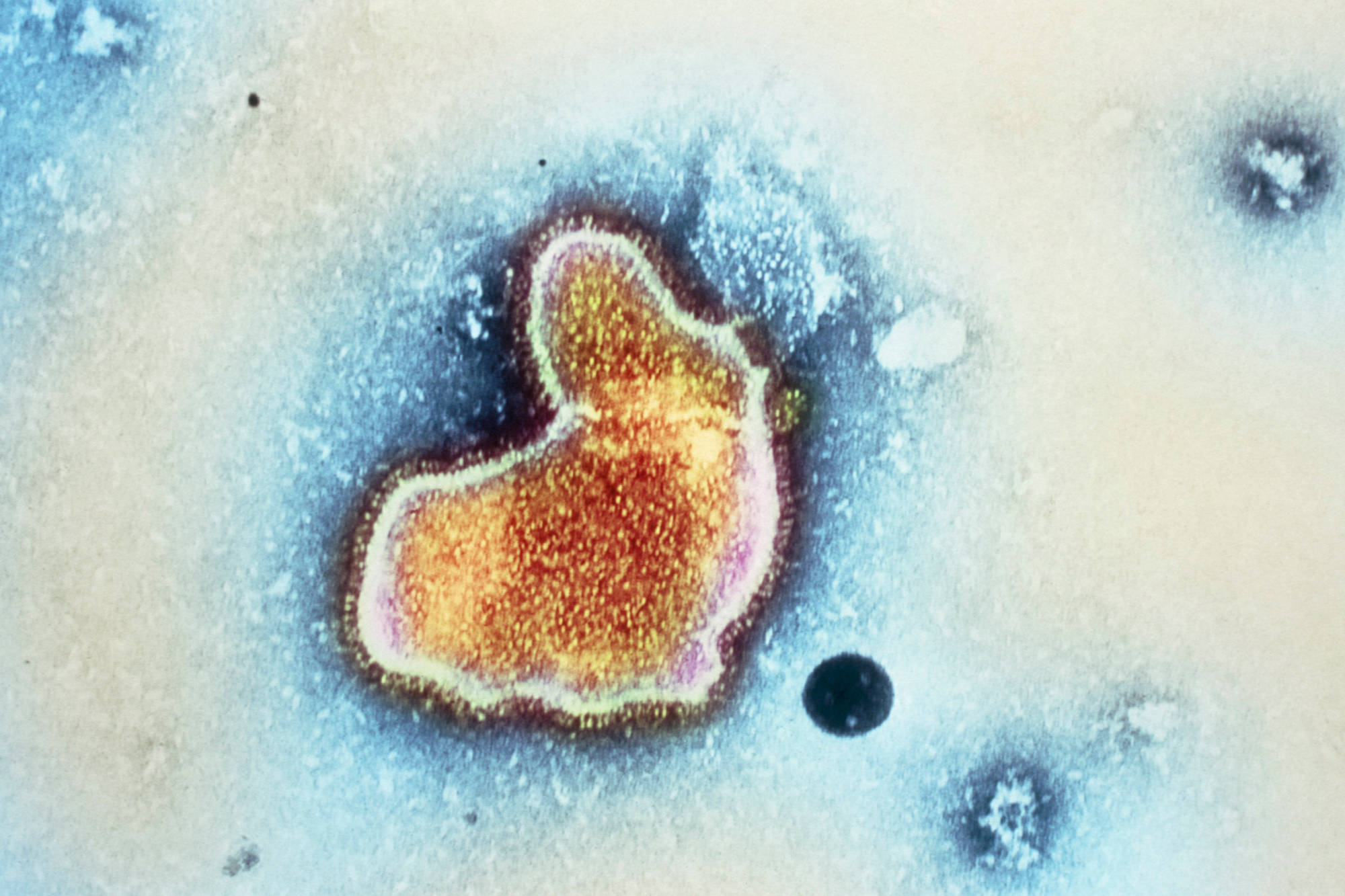
By the early 1960s, researchers realized that RSV was everywhere — leaving older children sick with sniffles, and sometimes landing infants in intensive care. When babies were hospitalized with pneumonia, or developed severe inflammation in the branching passageways of their tiny lungs, they often turned out to be infected with the virus. For some of those infants, the disease was fatal. (Adults catch RSV, too, but at the time researchers thought their symptoms were generally mild. Only years later would they realize RSV’s heavy toll on the elderly.)
In 1962, when the federal government launched a program to develop vaccines for common respiratory illnesses, RSV topped the list of priorities.
In 1962, a formaldehyde-inactivated RSV vaccine was tested in children for the first time, by doctors at Children’s Hospital in Washington, D.C. It flopped.
The cutting edge of vaccine development in those days was an approach by Jonas Salk, the pioneering polio researcher. Salk had grown poliovirus in his lab and then exposed it to formaldehyde. The chemical left the poliovirus impotent but intact. When the now-harmless virus was injected into a human body, the immune system learned to recognize and fight it.
Public enthusiasm for the vaccine was high, and parents signed up more than 1 million children to receive an experimental version of the shot. Polio cases plummeted.
Chanock and his colleagues decided to use the same technique to make a vaccine for RSV. In 1962, a formaldehyde-inactivated RSV vaccine was tested in children for the first time, by doctors at Children’s Hospital in Washington, D.C. At least 54 children received doses.
It flopped. The vaccine didn’t seem to provoke much of a response from the children’s immune systems, and, during the RSV season that winter, 21 of the subjects caught RSV. Ten of them ended up in the hospital — an unusually high rate. Years later, Chanock and several colleagues acknowledged realizing, even then, that the situation “was possibly outside the range of normal.” But it’s not clear if anyone at the time suspected that the vaccine itself could have caused those children to become so sick when they caught RSV.
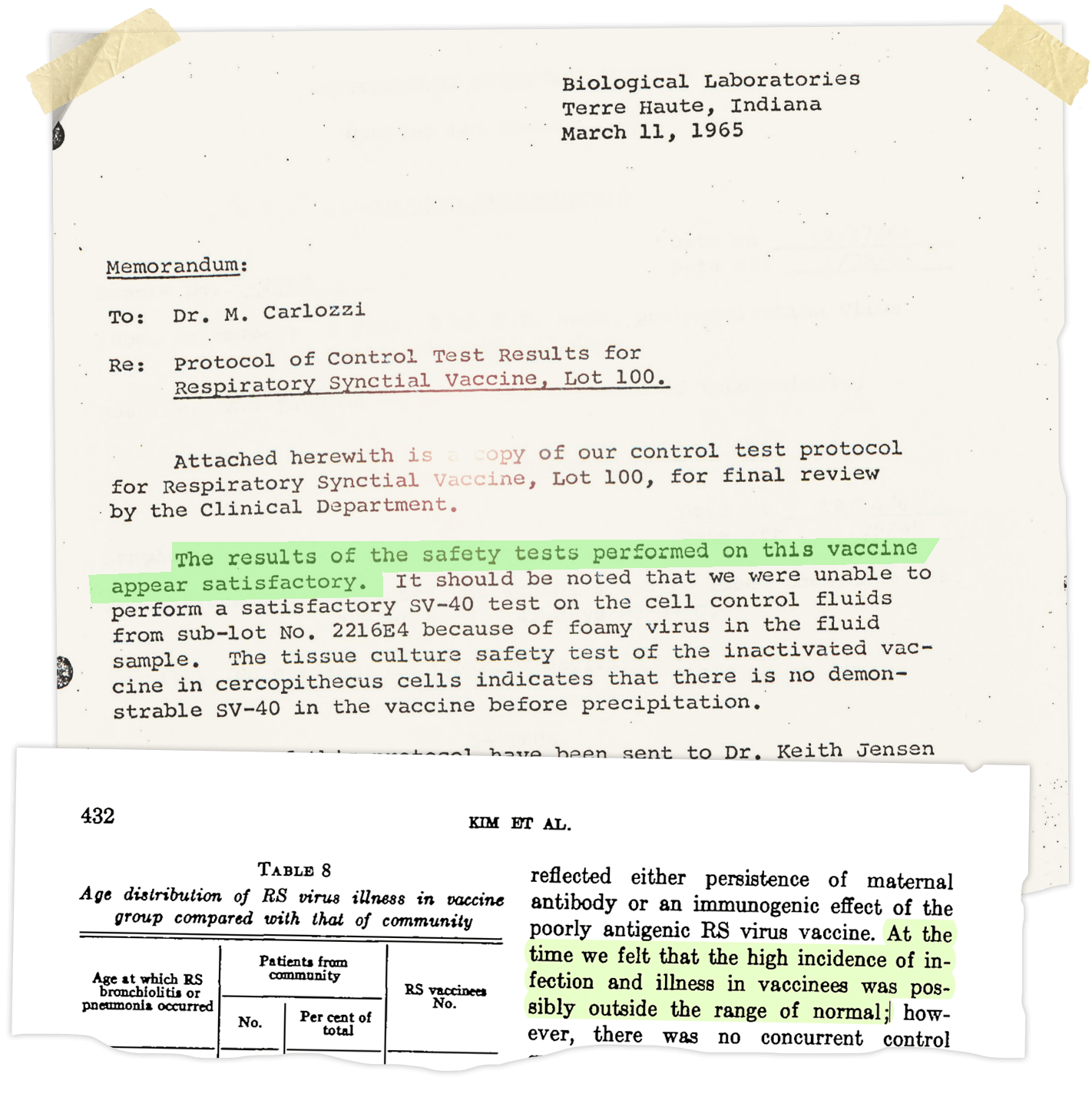
The researchers pressed on with Salk’s technique. Soon after the 1962 trial, Chanock and his team enlisted Charles Pfizer & Company to manufacture a more concentrated version of the same vaccine. The resulting experimental batch was called Lot 100.
In March 1965, a memo circulated within Pfizer. The researchers had injected the Lot 100 vaccine into four mice, four guinea pigs, and 25 cynomolgus monkeys. The animals stayed healthy. “The results of the safety tests performed on this vaccine appear satisfactory,” the memo concluded, noting that the details would be forwarded to the Vaccine Development Branch at the National Institutes of Health.
To get to his new job at NIH, Joseph Hambrick makes a reverse commute, traveling out of the dense rowhouses of the District of Columbia and into suburban Bethesda.
Hambrick works as an orderly in the Clinical Center, a specialized hospital where all the patients are participants in research studies. When surgeons need a certain body part shaved before a procedure, Hambrick wields the razor. He wheels patients to and from the operating room, and after procedures, he cleans the OR. Wearing scrubs and a mask, he gathers up bloody linens; with a mop and chemical soaps, he washes the floor.
When he starts work in the spring of 1964, Hambrick’s salary is around $3,620 per year — roughly $36,000 today, adjusting for inflation. He is the sole breadwinner for a family of five. To make ends meet, he works a series of second jobs in addition to his roles at NIH: one at a chicken restaurant, another behind the seafood counter of a Giant supermarket.
Hambrick grows close to another orderly at the hospital, Ernest Gabriel, a Korean War veteran from Richmond, Virginia. At the hospital, Gabriel would later recall, virtually all of the doctors, nurses, and administrative staff were White. The orderlies, truck drivers, janitors, and warehouse workers were mostly Black.
At one point, Hambrick persuades Gabriel to join a group, called All Concerned Employees, that aims to fight for better conditions for Black workers. They start by advocating for the laborers in the NIH hospital laundry room, where pay is low and breaks are short. “They worked like dogs,” Gabriel would later recall. White managers ring bells to summon their Black employees, “like they did slaves.”
The Hambricks eventually settle into the basement of a modest rowhouse in northwest D.C., near Upshur Park, owned by an elderly relative known as Mama Heard. The house has a deep porch and bay windows. A set of outdoor steps lead down to the basement, which is divvied up into rooms by hanging curtains.



In the summer of 1965, Janet King gives birth to another son, and she brings him home to the neighborly block of Willard Street — just 2 miles south of the Hambricks along Washington’s busy 16th Street corridor. The boy’s name is Victor Marcellus King, but everyone calls him Moochie. Janet will eventually marry his father, Thurondie Chisholm.
Victor is a happy baby, quick to walk, quick to talk, and often gravitating toward his older brother, Darius.
One day, Victor, now around 1 year old, takes his mother’s red and white roller-skating bag and puts something in it — maybe a diaper? He then sits on his butt and scoots down the long stretch of stairs to the sidewalk along Willard Street. As Janet and Darius watch on, amused, Victor marches off down the block, dragging the bag along with him. “I don’t know where the hell he was going,” Darius will recall decades later.
Before Victor can get to the corner, Janet sends Darius out to bring his baby brother back. “She wasn’t letting him go far,” Darius remembers.
Documents suggest that Janet brings Victor to a clinic run by Children’s Hospital, which has struggled financially. Newspaper reports describe the hospital offering care to 52,000 pediatric patients who are mostly unable to pay for it, nudging the facility toward bankruptcy.
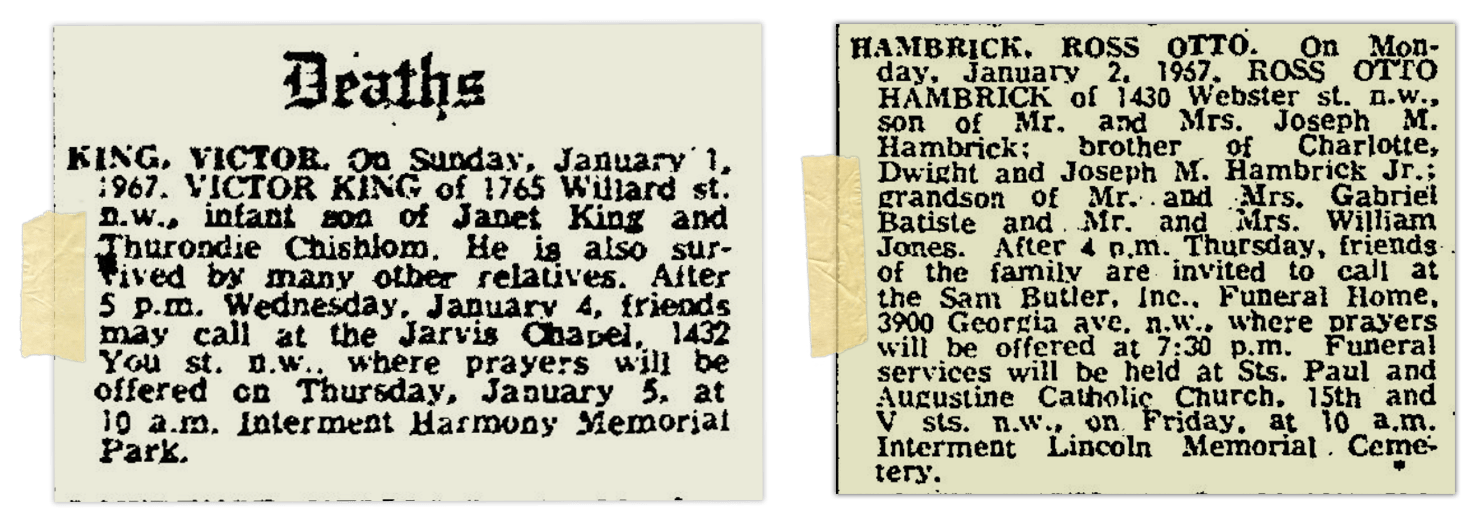
On Oct. 8, 1965, around two months after Victor King’s arrival, Emily Hambrick gives birth to another son. They name him Ross Otto Hambrick, and the family often calls him by both his given names: Ross Otto.
Emily is now 23 years old, living in a basement apartment with four children under the age of 5, a thousand miles from her family in Louisiana. Joe is holding down multiple jobs, including his role at NIH, to support the family.
Ross Otto is happy and healthy, with plump cheeks and a full head of hair. Emily puts the hair in braids, but Joe objects. When she cuts the braids off, Emily sets one or two aside for safekeeping.
The Hambricks and the Kings don’t know each other, but their lives in the working-class milieu of northwest Washington share similar contours. And so, like many other Black parents in the city do with their own children — and as Janet King does with Victor — the Hambricks take Ross Otto to one of the community clinics run by Children’s Hospital.
In December 1965, Chanock and his team launched a clinical trial of the Lot 100 respiratory syncytial virus vaccine. Ultimately, children would receive the vaccine at four different sites — two of them in Washington, where Chanock’s team played a more active role in the research. The participants, ranging from infants to children as old as 9, were sorted into groups: Some would get the Lot 100 vaccine, and some would not. The researchers made arrangements to track many of the children, performing occasional blood draws and testing them when they got sick.
One of the research sites was Junior Village, a campus of low-slung barracks in the far southern corner of Washington, near the Potomac River. It was operated by the District of Columbia as a foster care site. More than 90 percent of the residents — or inmates, as one document calls them — were Black. In an article published in Harper’s Magazine that year, the writer J.W. Anderson described the facility as “too big, too crowded and desperately understaffed.” Small children wandered up to Anderson during his visit, seemingly eager for human touch. “The little ones,” he wrote, “anxiously call everyone ‘Mommy’ and want to be held.”
Junior Village was sometimes described as an orphanage, but many, if not most, residents had living parents. Some of those parents were incarcerated; others had been deemed unfit to care for their children. Around half of Junior Village residents, according to Anderson, were there simply because their parents could not afford to support them.
The facility was shuttered in the 1970s amid allegations of widespread rape and abuse, including reports that staff had locked up and drugged children.
For researchers, Junior Village offered an ideal site — a captive population of young kids who could be monitored for infections. “Junior Village was incredible,” Chanock told an oral historian in 2001, describing more than a decade of research there studying outbreaks of RSV, parainfluenza, and other pathogens. In return for supplying NIH scientists with a steady supply of young research subjects, Junior Village enjoyed some benefits, including staffing support from several NIH nurses.
During the RSV vaccine trial, 70 children at Junior Village, aged 6 months to 5 years, received doses of Lot 100 vaccine. They were monitored more intensively than children at the other three sites. A rectal temperature reading was taken daily to check for fevers; the children’s throats were swabbed, usually on Mondays and Thursdays.
After the Lot 100 trial was underway at Junior Village, teams of researchers, supported by federal grants, began administering the Lot 100 vaccine to children at two military bases. One was Fort Ord, a U.S. Army outpost set among oak forests on the central California coast. At least 219 children of base personnel, ranging in age from 4 months to 9 years, received one or two doses of the Lot 100 vaccine. A second site was at Lowry Air Force Base, an installation just outside Denver, Colorado. A total of 464 children there, all 6 months or older, were selected to get three doses of the Lot 100 vaccine.
Papers published after the trials don’t provide much demographic information about the military children, although one does record that the subjects in Colorado were mostly “from middle-class homes.” The military was integrated by the 1960s, and the population on bases was generally racially diverse.
A fourth research site was run by Children’s Hospital in Washington, where physicians recruited infants who received care in a community clinic run by the hospital. This trial included the youngest children, aged 2 to 7 months. For that reason, it later become clear, they were at the highest risk of complications from both RSV and the experimental vaccines.
Nearly all of those children — if not all of them — were Black and from low-income families. At least 31 children there received the Lot 100 vaccine.
Among them were Ross Otto Hambrick and Victor King, who received the first of three doses sometime in the winter of 1965-66. Victor was five months old when he received his first shot. Ross was two months old, making him one of the youngest children to participate in the entire research program.
Initial side effects were minimal.

At the time of the early RSV vaccine trials, research on human beings was almost entirely unregulated in the United States. Legally, researchers could inject experimental substances without consent, perform untested procedures, and even withhold treatments in the pursuit of new knowledge. Children were considered essential for much of that research.
In the early 20th century, according to medical historian Susan Lederer, some people described those young subjects as little heroes, standing on the frontlines of the fight against disease. But even then, some experiments — especially on institutionalized children — drew objections from concerned members of the public.
Codes of research ethics did exist by the 1960s. The Nuremberg Code, drafted in response to Nazi experiments during World War II, called on scientists to receive “voluntary consent” from research subjects, and to provide them with enough information “to make an understanding and enlightened decision.” The code also required researchers to take steps to protect “against even remote possibilities of injury, disability or death.”
The Declaration of Helsinki, finalized in 1964, entrenched many of those principles, although it skirted certain issues: Lederer’s research shows how American medical authorities blocked a provision in the Helsinki document that would have discouraged research on institutionalized people, including children in facilities like Junior Village.
Not everyone followed those codes. In 1966, as the Lot 100 vaccine trial was underway, a Harvard Medical School professor named Henry K. Beecher published a brief paper in the New England Journal of Medicine, alleging that ethical failures were widespread in American medical research. Physicians were frequently experimenting on people, he wrote, who would never have agreed “if they had been truly aware of the uses that would be made of them.”
“There is a belief prevalent in some sophisticated circles that attention to these matters would ‘block progress,’” he continued. But medical research, Beecher suggested, ultimately gains little from crossing certain lines.
Public trust in doctors at the time, and despite these concerns, was very high. “I think the dominant ethos was one in which people thought you don’t want to interfere with medical progress, that one could still trust physicians to act in the best interests,” Lederer said. The 1960s, she added, came at the tail of “a golden age of American respect for physicians.”
It is difficult to tell whether parents at Fort Ord, in California, provided any consent for their children to participate in the RSV vaccine trial; if so, a subsequent paper detailing the trial’s findings makes no note of it. At Lowry Air Force Base, the researchers later report, the children’s parents were told that the vaccine was experimental, and that other children had already received it “without immediate untoward effects.”
Those other children are presumably the ones at Junior Village and Children’s Hospital, who got their first doses of Lot 100 several months before the military children. At Junior Village, the project had undergone an ethics review, but, according to one document stored in the NIH archives, the researchers never obtained express consent from the children’s parents to give them experimental vaccines.
The document explained how officials justified that decision: The parents or guardians of children sent to Junior Village signed paperwork allowing the facility to provide immunizations to help keep their children healthy. Because experimental shots like the Lot 100 vaccine were intended to prevent disease, the officials argued, they were covered by that waiver, too.
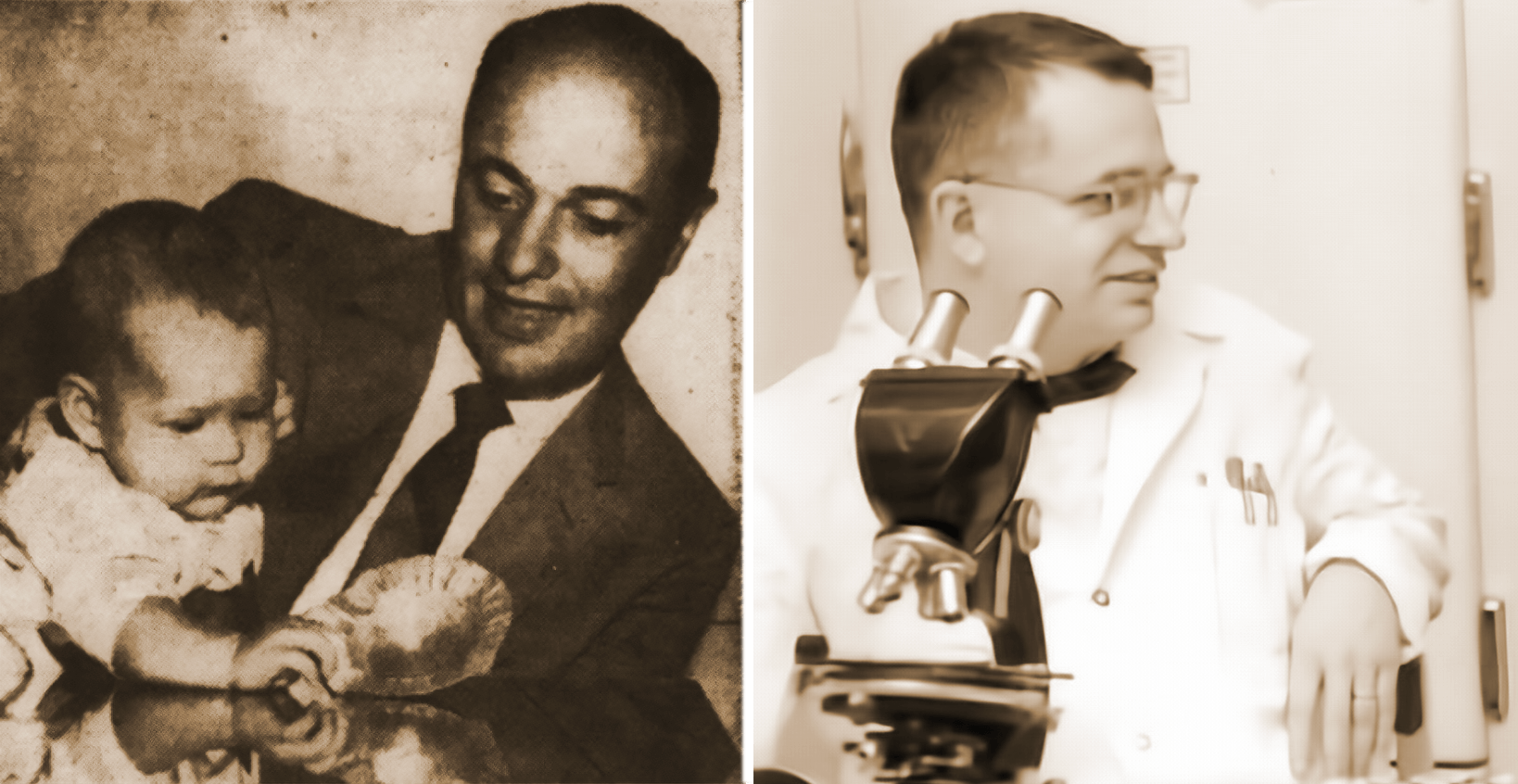
It was a rhetorical leap befitting an age of much more loosely regulated science. In the 1960s, Chanock and his colleagues conducted research on imprisoned people — a common practice at the time. Stephen Chanock recalls his father taking swabs from family members to hunt for new viruses, and giving his children unlicensed vaccines. “It was the Wild West,” Stephen said.
A few years after the trial, in an article in a scientific journal, the researchers who oversaw the study at Children’s Hospital indicated that they had received consent from the children’s parents. If that is true, a brief form housed at the National Library of Medicine appears to be the document those parents signed, although it is impossible to know for sure. A blank example of the form is included in a stapled packet of documents relating to the Lot 100 trial at the NLM, but signed forms are not included. (A spokesperson for Children’s National Hospital, as it is now called, said the institution does not retain research records from so long ago. Undark filed a Freedom of Information Act request with the NIH, seeking signed consent agreements for the RSV Lot 100 trial; that request is still pending.)
“If you wish,” the form obtained from the NLM begins, “your child may receive a newly developed vaccine intended to reduce the chance of getting colds, bronchitis and pneumonia.” The form goes on to say the vaccine has been “safety tested in many ways,” but does not provide specifics about the experiment.
The form also explains that the researchers “would like to follow your child for tests of how effective the vaccine is and what infections are eliminated.”

More than 50 years later, experts asked to review the document raised concerns. “This is woefully inadequate,” said bioethicist and writer Harriet A. Washington. The form, she noted, offers scant detail about what kind of safety testing the vaccine has undergone. (Indeed, the Lot 100 vaccine had seemingly never before been given to children, and there was no way for the researchers to know for sure that it was safe.)
Joseph Millum, a former NIH bioethicist, said the wording of the form implied that the vaccine was at least somewhat effective at preventing disease — something the researchers simply did not know. Consent forms have evolved dramatically since the 1960s, but Millum said that this doesn’t necessarily offer an excuse to the Children’s Hospital and NIH researchers. “You’re asking people to have their child injected with an experimental substance without giving them full information about whether that’s a good decision,” Millum said. “Saying that was the norm at the time doesn’t seem like a strong defense.”
Initial signs were encouraging. The children’s immune systems produced special proteins, called antibodies, that seemed likely to protect against RSV infections.
A sign of trouble appeared in the spring of 1966, a few months after the trial began. An RSV outbreak swept through the infants and toddlers of Washington. At least five children who had received Lot 100 from Children’s Hospital doctors caught the virus, and four of them ended up hospitalized — a strikingly high rate of severe disease.
“I am in shock,” Sharlette Hambrick said. “I always knew there was more to his death in my heart.”
It’s not clear, though, whether anyone sounded an alarm — or even noticed the pattern — in the spring of 1966. What is clear is that the researchers continued giving shots of the Lot 100 vaccine to children through that summer and into the winter of 1966. Around the time of the spring outbreak, Ross received his third, final dose of the vaccine. That summer, Victor received another dose as well. According to his subsequent bloodwork, Victor registered the strongest antibody response of anyone in that arm of the trial. His immune system was primed for a fight.
By that autumn, it seems, the researchers realized something might be wrong.
On a crisp morning in early November, Chanock attended a conference at the swank new headquarters of the Pan American Health Organization, in the Foggy Bottom neighborhood of Washington. Other researchers testing the Lot 100 vaccine also attended the conference, including Robert Parrott, the director of Children’s Hospital.
That morning, Parrott presented a paper to the assembled scientists; in a version of the presentation published a few months later, the team notes that the Lot 100 vaccine might be causing some children to grow severely ill when they caught RSV, rather than protecting them from the virus.
In the weeks after the conference, the team continued giving children doses of the Lot 100 vaccine.
Even today, RSV cases spike every winter, and in December 1966, right on cue, an outbreak began in Washington, D.C. Another Lot 100 vaccine recipient ended up in the hospital with RSV. Parrott and his colleagues reviewed the data and, on Dec. 29, 1966, concluded that it was time to stop the trial.

By that point, 16-month-old Victor King is already sick. The toddler begins showing cold-like symptoms in mid-December. His illness briefly improves, then worsens. When he’s admitted to the hospital on Dec. 30, he’s panting for air, taking 80 breaths per minute.
Hospital staff treat him with oxygen and antibiotics, but his breathing continues to grow more rapid. At 10:00 p.m. on New Year’s Eve, he’s taking more than 100 breaths per minute and running a fever. His lungs are failing.
Sharon, Janet King’s younger sister, is still a teenager. She goes out to a New Year’s party that night, but comes home early. When she gets back, an aunt is waiting for her. “Go to the hospital,” her aunt says. “Moochie took a turn for the worse.” Sharon immediately heads out, but it’s too late. “By the time I got to the hospital,” she later recalls, “he was gone.”
People in the family today say they find it difficult to believe that Joseph and Emily would have knowingly volunteered their child for the trial in the first place. “I’m sure they didn’t agree to it,” Brooks said.
An autopsy is conducted that afternoon. It determines that Victor had severe inflammation in his lungs, caused by RSV, as well as bacterial pneumonia that seemingly arrived on the coattails of the virus. During the autopsy, someone cuts away small pieces of his lungs, dips them in formaldehyde, and embeds them in paraffin wax to preserve the delicate tissue.
Ross Hambrick falls sick, too. On the morning of Dec. 30, his coughing intensifies, and he grows short of breath. Late that afternoon, he’s admitted to Children’s Hospital. His breathing grows more rapid; at times, he is taking nearly three breaths per second.
Joseph Hambrick’s family is Catholic, and when his sister Sallie hears that the boy is in the hospital, she realizes he needs to be baptized. As Ross’ condition worsens, she finds a priest to administer the sacrament. The ceremony, usually joyous, takes place in a hospital room. Sallie and her uncle Herman are present as Ross’ godparents. The child lies beneath a small canopy that allows doctors to supply him with extra oxygen. The priest touches water to his head:
“I baptize you in the name of the Father,And of the Son,
And of the Holy Spirit.”
Ross Otto Hambrick stops breathing at 10:30 a.m. on Jan. 2. That afternoon, a pathologist performs an autopsy. Like Victor, Ross is found to have experienced severe inflammation from RSV, coupled with bacterial pneumonia. He also has signs of sepsis. Someone preserves small samples from his lungs, too.
Sallie gets a call from her brother, who tells her that Ross has died. Not long after, Joseph drops off the other children — Joseph Jr., Dwight, and Sharlette — at the apartment Sallie shares with her sister.
Years later, Sharlette, who was 5 years old at the time, would remember her parents rushing to the hospital: “And then we were told our brother was in heaven.”
According to his obituary, Victor was buried on Jan. 5, 1967, at a cemetery in Maryland. Darius, then 5 years old, was not permitted to go, because his grandmother believed he was too young to attend.
Sharon King — now Sharon Johnson — was 19 years old when Victor passed, but she says she recalls a conversation among family members about the circumstances surrounding her nephew’s death: that he had been part of some kind of medical trial; that in recompense for his loss, the family had received funeral expenses, plus $1,000 — around $9,000 today, adjusting for inflation.
If anyone ever told Joseph and Emily Hambrick that the Lot 100 vaccine was likely responsible for Ross Otto’s death, or offered them some kind of compensatory payment, it would appear they did not talk about it. Until contacted by Undark for this article, Ross’ surviving siblings had never heard that a clinical trial likely contributed to his death. Neither had his godmother, Joseph’s sister Sallie Brooks. Nor had other relatives and friends who were close to Ross’ parents.
People in the family today say they find it difficult to believe that Joseph and Emily would have knowingly volunteered their child for the trial in the first place. “I’m sure they didn’t agree to it,” Brooks said. “I’m sure they knew nothing about it.”
According to his obituary, Ross’ funeral took place at the Saints Paul and Augustine Catholic Church, a historic hub of Black Catholic life in the District. An aunt, Esther Mason, remembers seeing the three surviving children — nearly 6-year-old Joseph Jr., 5-year-old Sharlette, and 2-year-old Dwight — at the service. Sharlette now says she wonders if the funeral marked the beginning of Emily’s deep vigilance over her remaining children. After that point, Sharlette says, her mother would not let her children out of her sight.
Brooks, Joseph’s sister, was particularly close to Emily. She recalls trying hard to hold herself together during the funeral, for the sake of her sister-in-law. “I didn’t want to make it any harder on Emily,” she said. But she couldn’t do it. Feeling too upset to stay, she left the room.
On the day after Ross died, Parrott, director of Children’s Hospital and a leader of the study, sent a long letter to a senior official at the NIH. About a week earlier, Parrott explained in the letter, he and his colleagues concluded that researchers should immediately cease giving doses of Lot 100. The deaths of Ross and Victor only reinforced the urgency of this request.
Over the course of four typewritten pages, Parrott laid out evidence that the Lot 100 vaccine could be causing children to get more severe cases of RSV. He also speculated that, perhaps, something similar had happened during the RSV vaccine trial in 1962 that ended with 10 hospitalized children. “The above findings were entirely unexpected,” Parrott wrote.

On Jan. 13, Parrott and another physician leading the study, Hyun Wha Kim, wrote letters to parents whose children had received doses of the Lot 100 experimental vaccine, but not yet caught RSV. A copy of one such letter, addressed to the parents of a toddler named Adam, is housed in the National Library of Medicine. The language is stilted. “This is to alert you to the fact that we know that the virus infection against which Adam received a possible vaccine is present in the neighborhood,” it began. “We also now believe that this vaccine is not preventing infection.” Parrott and Kim urged Adam’s parents to notify the hospital immediately if he showed any symptoms of a cold or cough.
“Some children can become very ill rapidly with the virus which is in the neighborhood,” the letter continued. Parrott and Kim did not explain that the Lot 100 vaccine itself had likely made the children more susceptible to severe disease.
In a letter to the NIH the next week, Parrott was more direct. Compared to those who had not received Lot 100, “infants who have received this vaccine,” he wrote, “tend to have a more serious clinical response to respiratory syncytial virus infection.”
Parrott enclosed a special protocol that the hospital had developed in case more recipients of the Lot 100 vaccines fell ill. The children were to be placed on bed rest, assigned a private duty nurse, and given throat and rectal swabs to check for viruses. “In anticipation of resuscitative measures,” the protocol advised, hospital staff should record the weight and height of each child at the time of admission.
It’s unclear if the protocol was ever used. But more than half of the subjects in that arm of the trial did grow seriously ill. In total, 18 children in the Children’s Hospital trial ended up hospitalized with RSV after receiving the Lot 100 vaccine, compared to just one in the control group. Other than Ross and Victor, they all appear to have recovered.
Children grew sick at other study sites, too. At Fort Ord, nine children were hospitalized with serious cases of RSV. At Lowry Air Force Base, in Colorado, 11 children suffered severe enough illness to be hospitalized; one of them, according to a subsequent report, required “major therapeutic measures, including prolonged assisted ventilation,” but ultimately survived. At Junior Village, five children were hospitalized, and one ended up in critical care. According to the research team, she made a full recovery.
Just months after the Lot 100 trial began, the Surgeon General began requiring a larger portion of American clinical research to receive ethical review, according to Behind Closed Doors, an academic study of ethics review in American research. But real reform only came after 1972, when a reporter for the Associated Press broke the story of the Tuskegee trial. For 40 years, U.S. government physicians working at the Tuskegee Institute in Alabama had failed to treat 399 Black men for syphilis. Instead of curing them with a simple antibiotic, the doctors allowed syphilis cases to linger in order to study the long-term effects of the disease.
The Tuskegee revelations caused a national uproar. In Washington, wheels began to turn. Congress enacted a more rigorous system for overseeing research in 1974 — one with legal teeth. The lawmakers also created a commission to review U.S. research. The group’s final report charged scientists with ensuring that vulnerable groups are not “systematically selected” for research “because of their easy availability, their compromised position, or their manipulability.” And it laid out expectations for informed consent. Subjects needed to understand the risks of an experiment, and to know that their participation was voluntary.
Whether and how much these reforms weighed on the Children’s Hospital researchers, particularly in light of the failures of the Lot 100 experiments, remains unclear. But the deaths of Ross Otto Hambrick and Victor King were only sporadically acknowledged by the principal investigators, at least in public. In 1969, Parrott joined six other scientists, including lead investigators Robert Chanock and Hyun Wha Kim, in co-authoring a paper published in the American Journal of Epidemiology describing the Children’s Hospital trial. Deep in the report, a few paragraphs discussed the deaths of a 14-month-old and a 16-month-old child, including details from their autopsies.
The NIH Record, an internal newspaper, seemingly made no mention of the trial, although it occasionally included items about the RSV research program. When The New York Times published a short article about Chanock’s RSV research in 1968, the reporter brought up the Lot 100 vaccine trial but did not mention any fatalities, instead implying that the ill effects had been short-lived.
A few years after the end of the Lot 100 trials, Chanock gave a talk at Georgetown University, analyzing the research at length. According to a transcript of the talk, now housed in his papers at the National Library of Medicine, he did not mention that two subjects died. Three decades later, discussing the vaccine’s failure with an oral historian, he again did not bring up the two fatalities.
Still, the failure of the Lot 100 experiments — including the deaths of Ross Otto and Victor — did cast an immediate pall over Chanock’s laboratory at the NIH. Peter F. Wright, now a pediatric infectious disease expert at Dartmouth, had joined Chanock’s team as a young doctor in 1968. “It was a shadow over the lab,” he now recalls. The failure of the trial, Wright says, “certainly permeated the thinking and even the psyche” of Chanock’s lab.
Robert Parrott’s oldest daughter, Maureen — now a pediatrician herself — says her late father often talked with her about his work, but she’s not sure he ever mentioned the Lot 100 trial.
In a 1970 letter to an NIH official, Parrott wrote about the hospital’s ongoing obligation to care for the survivors of the Lot 100 trial, as well as other children who had participated in vaccine research there. “These infants and children,” he wrote, “in a very real way, are in the front lines of the battle against respiratory illness.”
After Victor dies, Darius is once again the only child. He’s 5 years old, and he often hears his mother crying in the night — though few of the adults around him speak to him about Victor. An important exception is his grandmother, who sometimes points out the bay window of their home, after evening prayers, and tells Darius to look at a twinkling star in the sky. “That’s your little brother looking down on you,” Darius recalls her telling him, “making sure you’re okay.”
Janet King marries Thurondie Chisholm shortly after Victor passes. They have two more sons, and then a daughter. Janet and Thurondie, Darius says, become more religious. In 1976, as economic conditions in the neighborhood worsen, the family joins an exodus of Black families that could afford to leave, heading for the Maryland suburbs.
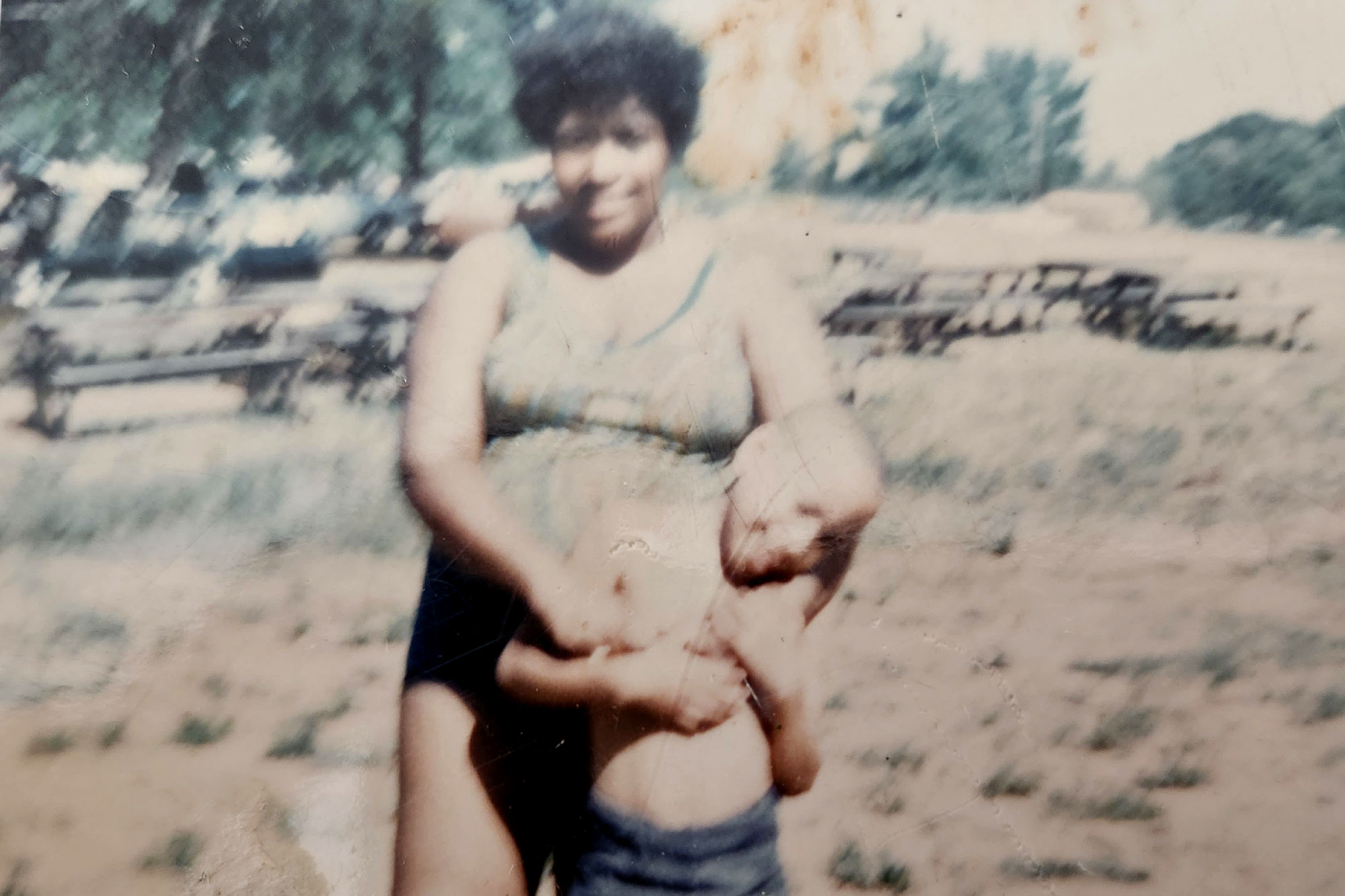


Not long after Ross dies, the Hambrick family moves out of Mama Heard’s basement to a house on Roxboro Place, in northwest D.C. Emily and Joseph have another son, Ron, in 1970, and Joseph pursues further education, becoming a registered nurse and earning a bachelor’s degree from Marymount University, where he graduates near the top of his class. He continues working at the NIH for most of his remaining career. It’s unclear whether he ever learns that some of his fellow employees at the agency had designed the experiment that may have killed his young son.
Emily rarely speaks of Ross. Following his death, she starts going to church four or five days per week, and she becomes intensely protective of her remaining children. At swim lessons, the other parents drop off their kids off and leave. But Emily waits outside the pool, her face against the fence, watching her children learn to make their way safely in the water.
As a young adult, Joseph Jr. considers changing his first name to Ross, to honor his brother. But when he brings up the idea with his mother, she asks him to reconsider. Another time, Joseph Jr. finds himself noticing — with a kind of intense, mysterious interest — a cemetery that he passes whenever he drives out of the District toward Maryland. He asks Emily about it, and she tells him that Ross is buried there. One day, they visit the cemetery together, but she shares very little. “She was struggling, and she didn’t want to talk about it,” he recalls. “So I let it go.”
Around 1977 or 1978, a young infectious disease researcher at the NIH, Gregory Prince, paid a visit to the pathology department at Children’s Hospital. He asked if they had retained samples from the autopsies of the two children who died during the Lot 100 trial. Understanding what happened, he and his colleagues believed, was crucial for safely taking next steps in RSV vaccine research.
“Pathologists are pack rats,” Prince said in a recent interview. “They don’t throw anything away.” Indeed, the Children’s Hospital pathology department still had the boys’ samples. A pathologist there allowed Prince to take some of the tissue back to the NIH. With a fine blade, a technician sectioned off pieces of the paraffin blocks, which contained tiny pieces of lung tissue from Victor and Ross.
Darius, Victor’s brother, suggested that the circumstances of his brother’s death follow a pattern that feels familiar for many Black people. “We’re not surprised,” Darius said. “It’s just American history.”
“The blocks had no names on them,” Prince recalled. “They just had numbers of what the autopsy case was. There was no violation of identity.” In the dozens of scientific papers that have since discussed their case, the children go by other names: Sometimes they are referred to as the 14-month-old and the 16-month-old, for example. In another instance, they are simply known as ERD1 and ERD2, using an acronym that stands for “enhanced respiratory disease.”
As he worked with the lung samples, Prince stained small pieces of the tissue with a special dye, and pressed them between pieces of glass. Through a microscope, the delicate tissue appears as a psychedelic swirl of purple and white.
It’s not clear if anyone in the families realized back then that those samples existed, or that they were being used for research, but by the 1980s, the enhanced RSV disease that killed Ross and Victor had become the subject of more extensive scientific research. At NIH, Prince dug up old vials of Lot 100 vaccine and injected it into cotton rats, which respond to RSV similarly to human beings. Other laboratories were also documenting the way the rats’ immune systems cope with RSV.
The failure of the Lot 100 vaccine had made it difficult to receive the greenlight to develop new RSV vaccines, effectively freezing the field. Researchers hoped that, by discovering what went wrong, they would know what to avoid in the future, and be able to produce a safe, working vaccine. Through it all, the subjects of the Lot 100 trial loomed large. “All of us are very aware of those trials, and are very aware of the terrible outcome, and the incredible responsibility that all of us have,” said Ruth Karron, a physician and researcher at Johns Hopkins University who began studying RSV in the early 1990s.
At some international meetings in her early days in the field, where 200 or more RSV researchers gather to talk about their work, a substantial portion of the meeting seemed to be presentations on research aiming to understand the enhanced disease caused by Lot 100, Karron said.
Over the years, countless papers mentioned the deaths of two boys, some exploring the details of their medical histories in depth, though their names and personal histories remained shrouded. “We’ve been talking about these children for years and years as the basis for all the work we were doing,” said Barney Graham, a prominent RSV researcher who began his career, in the 1980s, by studying the enhanced disease.
On one autopsy report that circulated among scientists, each name is redacted by a thicket of black marks. It’s a vital step to protect each child’s right to privacy — part of researchers’ obligation to build a barrier between a person’s medical data and a person’s identity.
At the same time, such redactions had a curious effect: Even as their lives and deaths loomed over an entire field of research, Victor and Ross disappeared. Speaking with Undark in 2023, few RSV researchers knew anything but basic details about the lives of the children who helped to shape their field. Some scientists described the boys, incorrectly, as residents of Junior Village, the foster care facility.
The crucial breakthrough for the development of safe, effective RSV vaccines was a trick of sight — a feat of biology and engineering that made it possible to gaze upon the previously unseen.
The surface of the RSV virus is studded with something called the F protein, which allows the virus to enter human cells. The problem for researchers is that the F protein is unstable: The slightest trigger can cause it to change into a new shape. For years, scientists didn’t realize the unstable form of the protein even existed. “That’s why science, I think, is a faith exercise,” said Barney Graham. “You have to at least imagine that things can be real that are not seen.”
Starting in 2008, structural biologist Jason McLellan, Barney Graham, and other scientists at NIH began the slow work of trying to freeze the protein in place, then catch a glimpse of it. In 2013, they published a breakthrough: Using immune system proteins that glom onto the viral F protein, they had managed to fix it in place, then figure out its exact shape.
That discovery opened up new avenues for vaccine development. By the late 2010s, researchers were working on several potential RSV vaccines that consisted, essentially, of a stabilized form of the F protein. Other researchers were developing vaccines that consisted of active, but weakened, strains of RSV.
At the same time, scientists now have a much better understanding of what went wrong with the original Lot 100 vaccine. That understanding came, in part, from studying the lung tissues of Ross Hambrick and Victor King.
One day in 2015 or 2016, a package arrived at the University of Pittsburgh laboratory of Jay Kolls. Inside it were two slides, one containing tissue from Ross, the other from Victor.
Their samples had traveled far since that day in the late 1970s when Gregory Prince visited the pathology department of Children’s Hospital. Prince eventually shared them with Fernando Polack, then a scientist at Johns Hopkins University in Baltimore, who took them with him to a subsequent post at Vanderbilt. Now they had arrived in Pittsburgh, where Kolls planned to use new techniques in RNA sequencing to study, in even greater detail than before, the precise way the boys’ immune systems had responded to RSV after receiving doses of the Lot 100 vaccine.
Today, at least four peer-reviewed scientific papers — including a 2021 article co-authored by Kolls (now at Tulane University), Polack, and other scientists — include analyses of the boys’ autopsy samples. That work, in addition to research on animals, has allowed scientists to explain what went wrong during the Lot 100 trial.
When a person catches RSV for the first time, their immune system learns to produce proteins, called antibodies, that are able to stick to the virus and trigger a process that will eventually destroy it. The antibodies fit certain places on the F protein precisely, like a key cut to fit a lock. When the virus comes again, even years later, the body knows how to produce those antibodies.
Lot 100, though, changed the shape of the hair-trigger F proteins on the virus’ surface. The immune systems of the youngest children — the ones who had never gotten RSV before — learned to produce a misshapen key. When RSV showed up, months later, those hapless antibodies were able to stick to the virus, but not destroy it. The body kept throwing more and more weapons into the fray, which damaged the lungs, rather than helping them out.
The Lot 100 vaccine also taught the children’s immune systems to respond to RSV as if it were an allergen or a parasitic worm, rather than a virus. When the children later caught RSV, that immune response produced something that resembled an allergic reaction. For the tiniest children, with the most delicate lungs, the effects were especially dangerous.
Understanding all of this did more than solve a decades-old medical mystery. It also offered researchers a set of warning signs that have proven useful for vaccine development. Today, researchers know, in much more detail, the kinds of immune system activity that signal a potential repeat of the Lot 100 tragedy.
In 2019, the World Health Organization pulled together a group of experts to write guidelines for developing safe RSV vaccines. Their report, published in 2020, draws on research examining Victor and Ross’ tissues, as well as research on blood samples from participants of the Lot 100 trial.
Judy Beeler, an FDA medical officer and the chair of the WHO committee, described the document as “a useful, useful guideline to help maneuver a safe vaccine into clinical trials” for the youngest children. Beeler, who helps evaluate RSV vaccines at the FDA, said she uses the guidelines regularly.
Dozens of RSV vaccines are now in various stages of development. Some will be widely used this autumn. “These are really, really well-studied vaccines,” said Peter Openshaw, an RSV researcher at Imperial College London. “You couldn’t possibly launch a vaccine trial like you did back in the 1960s these days. We have to do so much preclinical work, so much work in animal models before you ever go into humans.”
Robert Chanock did not live to see the arrival of the RSV vaccines he spent decades working to create. He died in 2010, suffering from Alzheimer’s disease.
As a young researcher, in the 1980s, Barney Graham spent years studying the kind of enhanced disease that was caused by the Lot 100 vaccine. “I think you can draw a direct line from the sacrifice those children made to what’s finally happening now,” he said in a recent interview. The virologist, who is one of the world’s most prominent RSV researchers, recently retired from a long stint at NIH, and now serves as a professor at the Morehouse University’s School of Medicine. (Like many RSV researchers, he also has a hand in pharmaceutical development, in his case as a consultant for GSK, Pfizer, and other drugmakers.)

“I think you can draw a direct line from the sacrifice those children made to what’s finally happening now,” said vaccine researcher Barney Graham. He is shown here holding a model of a mosaic nanoparticle influenza vaccine.
Visual: Amanda Andrade-Rhoades for The Washington Post via Getty
Graham and his colleagues’ techniques for studying the F protein have borne fruit far beyond the field of RSV research: They turn out to be key for the quick development of Covid-19 vaccines. The technology also underlies the fleet of new RSV vaccines that received approval this year.
During a conversation with Undark in March, Graham expressed concern about surfacing the story of Victor and Ross Otto now, on the cusp of RSV vaccine rollouts. Maybe, he wondered, it would induce fear of the new generation of RSV shots. In a conversation a few months later, though, he took a different view: “The thing is, there can be a backlash from telling the story, and there can be a backlash from not telling the story. It’s something we have to come to grips with, in biomedical science, of how to communicate bad news.”
In the March interview, Graham also brought up the possibility of some kind of recognition for the families involved in the Lot 100 trial. “Especially if these RSV vaccines can be successful, I would like to see some of these companies, and maybe the government, go back and do some repair work,” he said.
What would that look like? “Well, I don’t know yet,” Graham said. “I’m still thinking about how that could happen. But I think at some level, those children who experienced that need to be given some credit for what’s happening now.”
Reparations for harmful research on vulnerable groups are rare. There are exceptions: After the Tuskegee revelations, for example, the U.S. government set up a fund to compensate victims. Just this year, the company Thermo Fisher Scientific settled with the descendants of Henrietta Lacks, a Black woman who died of cancer in 1951; the company has profited from Lacks’ cells, which were taken without her consent.
Some cases are simply overlooked. “The problem with that history is it’s occult history,” said the bioethicist and writer Harriet A. Washington, whose award-winning 2006 book “Medical Apartheid” chronicled more than a century of unethical experimentation on Black Americans. That history, she added, is “still not taught.”
Other cases of research error and ethical transgression have probably been forgotten altogether. “I think one of the great mysteries about 20th century research is how many people actually die in research,” said Susan Lederer, the medical historian. “Because nobody collects it. It’s not in anyone’s interest to collect that data.”
There were several ways to find the names of the two boys who died in the Lot 100 trial, had someone been determined to look. First, their first initials and last names are listed, unredacted, in one of Robert Chanock’s green government-issue laboratory notebooks, now housed in a legal box in the National Library of Medicine. Second, an autopsy record published on the website of a scientific journal was inexpertly handled; the redaction of the boys’ names can be removed with a PDF image editor. And the autopsy itself contains precise dates of death, which line up with obituaries for Ross and Victor published in The Washington Evening Star.
The Office of NIH History also has complete lists of the participants in the Children’s Hospital and Junior Village trials — typewritten, in neat columns, on yellowing paper — but it will not permit researchers to record, copy, or use the names, because of concerns about patient privacy.
In May 2023, Undark messaged Sharlette Hambrick via LinkedIn, sharing a few details about the trial and asking if she was Ross’ sister. She wrote back the next day: Yes, Ross was her little brother. “I am in shock,” she added. “I always knew there was more to his death in my heart.”
Emily Hambrick, Ross’ mother, had died a few years before, as had one of Ross’ older siblings, Dwight. Joseph Hambrick Sr. was alive and back in Slidell, Louisiana, but he was suffering from advanced dementia. (He died in August, at the age of 79.) In interviews, none of the surviving Hambrick siblings said they knew that Ross had died in a medical trial; but they also didn’t express much surprise.

In subsequent conversations, Sharlette Hambrick sometimes described herself as split — simultaneously wanting some good to come out of the trial, but also angry at what happened to her brother, and hopeful for some accountability from NIH. “Is that possible, to have those two parallel universes?” she asked during a conversation in early August. (An agency spokesperson, Renate Myles, declined to make someone in NIH leadership available for an interview.)
Victor King’s mother, Janet King Chisholm, died in 2001. Victor’s father, Thurondie, died in 2021 from Covid-19. When reached by phone in May, Darius, Victor’s brother, was similarly unaware of the Lot 100 trial — though, in a later conversation, he suggested that the circumstances of his brother’s death follow a pattern that feels familiar for many Black people. “We’re not surprised,” Darius said. “It’s just American history.”
What roles race, class, or other considerations played in the selection of vaccine recipients is impossible to know — but two things are simultaneously true about the Lot 100 trial: It’s likely that most of the children to ultimately receive the vaccine were White, simply because the trials on the military bases were so large. At the same time, the first and youngest children to get the vaccine were Black.
In that context, had Ross and Victor not been Black children from working-class Washington D.C. families, they might well be alive today, in their late 50s, perhaps with children or even grandchildren of their own. “They weren’t up in Georgetown, you know, and Ambassador’s Row, experimenting on those children,” said Deirdre Cooper Owens, a historian who studies experimentation on Black Americans, after reviewing details of the trial.
For his part, Darius seemed inclined to look forward. “You can be upset about it, or just move on and try to make sure it don’t happen again. Who knows?” Darius added. “Maybe what my brother went through could help somebody. That’s always a way to look at it.”
Members of both families agreed to allow Undark to publicly disclose the names of the two boys they had lost to science: Ross Otto Hambrick and Victor King.
At least one estimate suggests that, by 2032, the global RSV vaccine market could be worth up to $10 billion — a prediction buoyed by waves of recent new drug approvals. In May 2023, the FDA approved the first RSV vaccine, for use in older adults. It’s called Arexvy, and it’s produced by the drugmaker GSK. The vaccine is essentially a form of the F protein that studs the virus’ surface, frozen in the shape that the immune system needs to recognize. In a trial, the vaccine cut seniors’ risk of getting a severe case of RSV by 94 percent.

Advertisements for the new RSV vaccines are proliferating, and some, like this one warning the RSV can cut lives short, are difficult for Sharlette Hambrick to watch.
Visual: Screen capture via Youtube
After Arexvy got approval in May, the company’s stock jumped. Weeks later, a similar vaccine, manufactured by Pfizer, received approval.
Also in May, a panel of FDA advisers endorsed giving the Pfizer vaccine to pregnant women. The mothers pass on some immunity to infants, cutting babies’ risk of a severe RSV infection. (In tests, women who received the vaccine were slightly more likely to have a premature birth, and while the connection may have had nothing to do with the shot itself, the pattern raised concerns from FDA advisers. The agency approved the shot this summer, but adjusted its timing to later in pregnancy.) And, in July, the agency approved a shot of immune proteins — a distinct therapy from the new vaccines that owe so much to the Lot 100 trial — that linger in a baby’s body for a few months, helping prevent severe cases of RSV.
At home, watching cable news, Sharlette Hambrick begins to see commercials for the new RSV vaccines for seniors. The experience can be jarring, now that she knows more about what happened to her brother. One commercial, from GSK, is especially hard for her to watch. The 30-second spot begins with images of older adults enjoying themselves — strolling through an art gallery, playing pickleball — while upbeat music plays in the background. Suddenly, the music stops, and the screen grows dark. “This moment could be cut short by RSV,” it reads.
“Basically, that was Ross’ life,” Sharlette said. “This cheerful, fun baby — all of a sudden, the world goes black, and he’s gone.”
Now in her 60s, the mother of a 21-year-old, Sharlette often finds herself reliving the events of Ross’ death through her parents’ eyes. “My parents were poor, young, and naïve,” she said. Emily and Joseph, she imagines, must have undergone their own plunge into silence.
Robert Chanock’s son Stephen is also a doctor, and also a senior scientist at NIH, where he leads a division that studies genetics and cancer. He works from a sunny, bonsai-filled office on the seventh floor of a blocky tower in the Washington, D.C. suburbs.
Stephen was in elementary school when the Lot 100 trial took place. He did not learn about it until he was in his 20s, studying medicine. A mentor, he recalls, asked him whether his father was different in some way around 1967. Stephen had no idea what he was talking about. Only later did he learn about the trial, and about the failure of Lot 100.
He describes his father as at once devastated by the outcome and cognizant that he could not dwell on it — that it was essential to dive back into research. His father, Stephen recalled, would say, “You can stand and look backwards in vaccinology. But you’re not going to go anywhere, because the infections are continuing to cycle or to spread.” The cost of a delay was just too high. “That’s just more people, more kids getting RSV and potentially dying,” Stephen said.
It’s a view that Stephen himself says that he has to adopt in his work as a pediatric oncologist. From the perspective of a patient, the success or failure of a treatment means everything. But the nature of medicine, Stephen suggests, sometimes requires physicians to adopt a broader perspective — one that permits them to move on when a patient cannot be saved or when a therapy fails.
“You have to come to terms with individual success and failure, and scientific success and failure,” he said. In his own work, he has lost patients who have largely succumbed to their cancer despite aggressive treatments — and despite his own best efforts to save them. “It’s very sad. You take a moment, but you’re on to the next thing, because there’s someone else who needs your help. You can’t sit there and say, ‘Oh, that was terrible.’ It was terrible. But you have to live with failure.”

On a Sunday afternoon in July, Sharlette Hambrick circles the Lincoln Memorial Cemetery, which straddles a pair of grassy hills just outside D.C. She is looking for the grave of her brother. Storm clouds stack up overhead as deer graze among the headstones, and dragonflies flick themselves through the humid air.
Ross, who has for so long been anonymous in the medical literature, is once again hard to locate: The cemetery staff, despite nearly a week’s notice, are unable to say precisely where he is, though their records confirm that he is buried here somewhere. Small children are often buried in clusters, in areas the cemetery refers to as a “babyland.” Some kids, like Ross, don’t have any marker. Burial for the youngest children is free, a staff member says, but a headstone is not.
Sharlette searches for half an hour. A brief sunshower comes and goes.
Near the back of the cemetery, cradled in the curve of a little suburban creek, is a plot of sloping grass. A few scattered plaques mark the graves of children, some of whom died in the ’50s, and ’60s. Sharlette is not sure Ross is in this exact spot, but it’s close enough, at least for now. “I know it’s wet,” she says, “but I’m just going to take a minute.” She sits in the rain-soaked grass.
Visiting the cemetery serves as a fresh reminder for Sharlette that once, for 14 months, her brother was in the world. “I thought it would be really, really sad,” Sharlette says as she leaves the cemetery. She pauses. “But it was more calming. It was more, like, wow, he actually existed. He was actually a little human being.”
Do you have information related to the development of RSV vaccines that you would like to share? Contact us confidentially at [email protected], or visit our Contact page for more ways to get in touch.








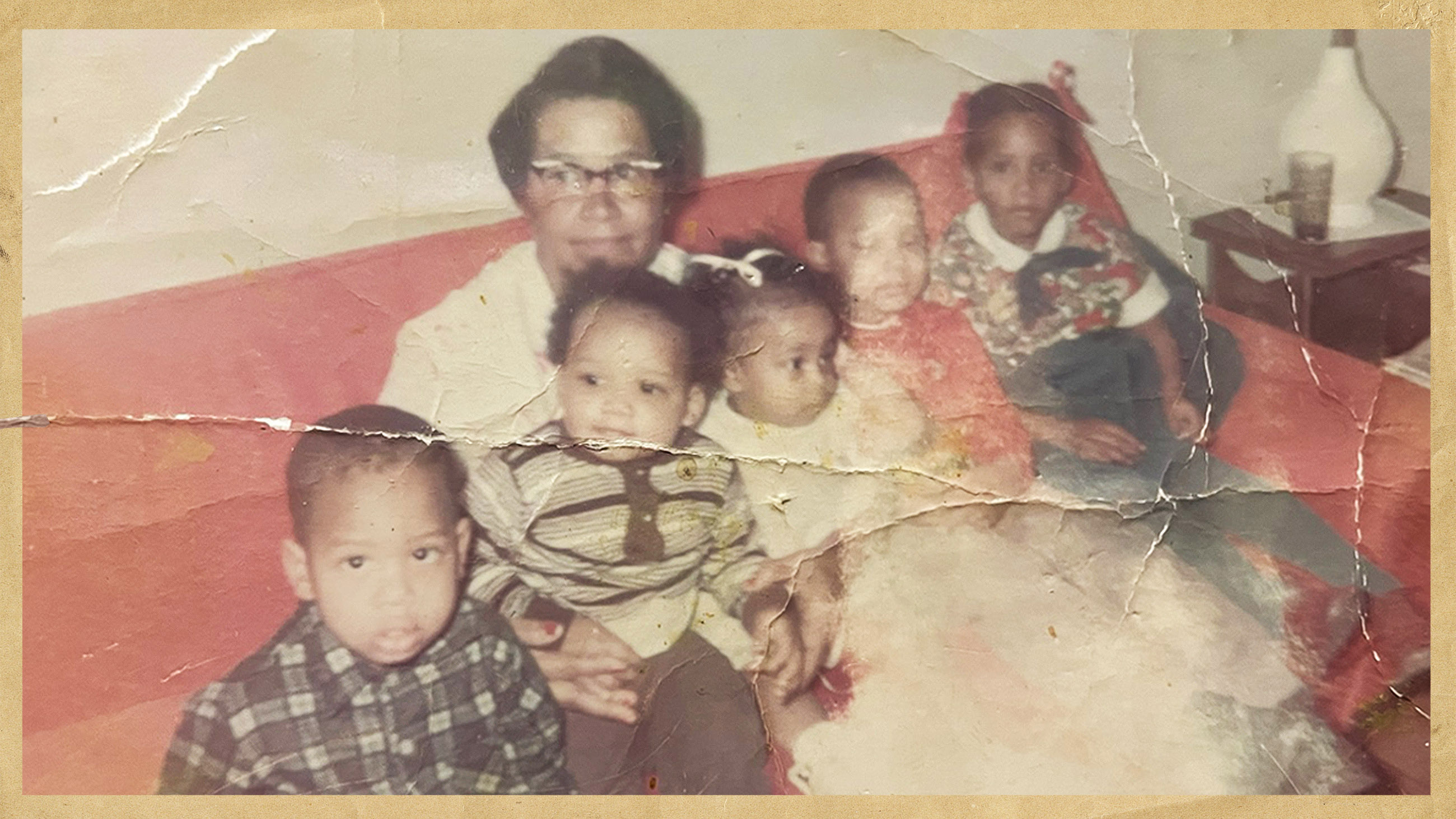
Comments are automatically closed one year after article publication. Archived comments are below.
From an infectious disease researcher: thank you so much for honoring Ross Otto Hambrick and Victor King. This should not have happened to them and should not happen again. Your article is a good reminder to look into the history of my own field of research. Science has to do better.
I got Long Vax from the COVID vaccine. The next time I got COVID – about 8 months later – I was much sicker than the first time I had COVID. I was hospitalized and now have a diagnosis of Long COVID. Not one medical person will acknowledge that the vaccine caused this. Will it be 50 years before they do? The more things change, the more things stay the same.
Thank you.
So scientists have learned nothing from Henrietta Lacks or her family. If scientists would like to regain the public’s trust, it needs to stop treating society’s margins like disposable lab rats.