By the 1920s, polio was menacing the world every year in different towns, cities, and countries. No one could predict where it would hit or how many it would fell. This virus that in the previous century had caused barely any disease was now creating terror, leaving paralysis and death in its wake. Doctors had little to offer — bed rest was the mantra of the era.

The accompanying article is excerpted and adapted from “The Autumn Ghost: How the Battle Against a Polio Epidemic Revolutionized Modern Medical Care,” by Hannah Wunsch (Greystone Books, 360 pages.)
The most devastating of all was when the virus attacked the nerves that controlled the muscles needed for breathing. Children would start gasping for breath. James L. Wilson, a resident (doctor in training) at Harvard during the 1920s, described the horror of caring for polio patients who were unable to breathe: “Of all the experiences that the physician must undergo, none can be more distressing than to watch respiratory paralysis in a child ill with poliomyelitis,” he wrote. “Using with increasing vigor every available accessory muscle of neck, shoulder and chin, silent, wasting no breath for speech, wide-eyed and frightened, conscious almost to the last breath.”
Well before paralytic polio arrived in the late 1800s, all kinds of problems caused people to stop breathing. Pneumonia was, of course, extremely common. But so were drownings and other accidents, such as gas poisoning. There was great interest in trying to figure out ways to resuscitate victims of drownings and other sudden events that led to death.
Normally, the body sucks air into the lungs when the diaphragm pushes down into the abdomen, and the ribs expand by using the chest muscles. This creates negative pressure inside the chest, forcing the lungs to expand to fill the void with air that rushes in through the mouth or nose, through the vocal cords, down the trachea and bronchi, and into the alveoli, the tissue of the lungs made up of tiny sacs of air. In the alveoli, gases diffuse between the air and the blood. Oxygen from the air gets delivered to the bloodstream, and carbon dioxide, the body’s waste, moves from the blood into the air.
In exhalation, the body just relaxes. The lungs naturally want to spring back, like a balloon after the knot holding the air in is untied. The diaphragm moves back up, the muscles of the chest wall relax, and the ribs return to their natural resting position. The air gets pushed back out of the trachea, through the mouth and nose.
As some understanding of anatomy and physiology developed, it became apparent there were two possible ways to get air into the lungs: increase the negative pressure around the lungs so that the lungs are pulled open by the external forces, the way normal breathing occurs, or push air or other gas directly into the lungs with positive pressure, like blowing up a balloon — an approach considered “unnatural.”
Many people experimented with both options through the 1800s and into the early 1900s without great success. A series of attempts by scientists in the early 1800s created artificial negative pressure by encasing the body in a box or tube and creating a seal around it, with a bellows or pump to then remove air from the chamber and create the negative pressure necessary to force the rib cage to expand and the lungs to open. None of these devices gained much traction as they were cumbersome, prone to leaks, and required someone to man the bellows or pump continuously.
Then children began to die from polio.
The first major breakthrough in the care of polio patients came from an unlikely source: a professor of industrial hygiene at the Harvard School of Public Health. Philip Drinker did not set out to change care for polio patients. What interested Drinker most were problems such as air pollution in factories and occupational injuries.
Drinker was born in Haverford, Pennsylvania, on December 12, 1894, the year of the first major polio outbreak in the U.S. He studied chemical engineering at Lehigh University before enlisting in the army in 1917. He was stationed overseas to work on the preparation of airplane fuselage coatings and after the war worked as an industrial engineer, focusing on understanding the health problems associated with air pollution in factories and shipyards and devising approaches to improve the safety of the working environment.
In 1921, Drinker took up a position at Harvard Medical School as an instructor in applied physiology, and then in 1923 became an instructor of ventilation and illumination at the new School of Public Health at Harvard.
In the early 1920s, more than 2,000 people in the U.S. died of “poisoning by gases and vapors” every year. So, in 1926, Drinker was appointed to a newly formed commission at Rockefeller Institute, charged with improving methods of resuscitation from gas poisoning and electric shocks. This work was supported by the Consolidated Gas and Electric Companies of New York, which had a vested interest in improving such technology.
Around this same time, Philip Drinker’s brother Cecil, along with a colleague, Louis Agassiz Shaw Jr., was tinkering with a method to measure the breathing of different animals, including the cat. They placed an anesthetized animal inside a plethysmograph — a metal box attached to a spirometer, which measures pressure, and a device to measure changes in volume.
One of Shaw’s questions, which he answered in a publication in 1928, was whether cats, and mammals in general, could breathe through their skin. In 1904, August Krogh had shown that pigeons, tortoises, frogs, and eels absorbed oxygen through the skin and gave off carbon dioxide — a phenomenon called cutaneous respiration. Eels did this so efficiently that they had no need for gills, but in tortoises and pigeons the exchange of gas was far too small to keep them alive. Mammals had not yet been studied.
Shaw sealed the cat in with its head sticking out. He could then measure how much air was going in and out of the lungs as the cat breathed by looking at a U-shaped tube half-filled with water. When the cat inhaled, the water level changed, and they could calculate how much air had entered the cat’s lungs. Because the plethysmograph made a seal around the cat’s body, Shaw could also determine if gas was being exchanged through the cat’s skin and not just through the lungs by sampling the gas within the box. He concluded that cats did not use their skin to breathe.
Drinker watched these experiments and seemed aware of possible applications to humans and their breathing. He conducted his own experiment by paralyzing a cat with curare, sealing it in the box, and then pumping air in and out by hand using a syringe, keeping the cat alive for a few hours. By pulling air out of the box, he created negative pressure as others had done before him, sucking air down the cat’s trachea and into the lungs. When he pushed air back into the box, the pressure increased again. The lungs could recoil along with the rib cage, causing the cat to exhale.

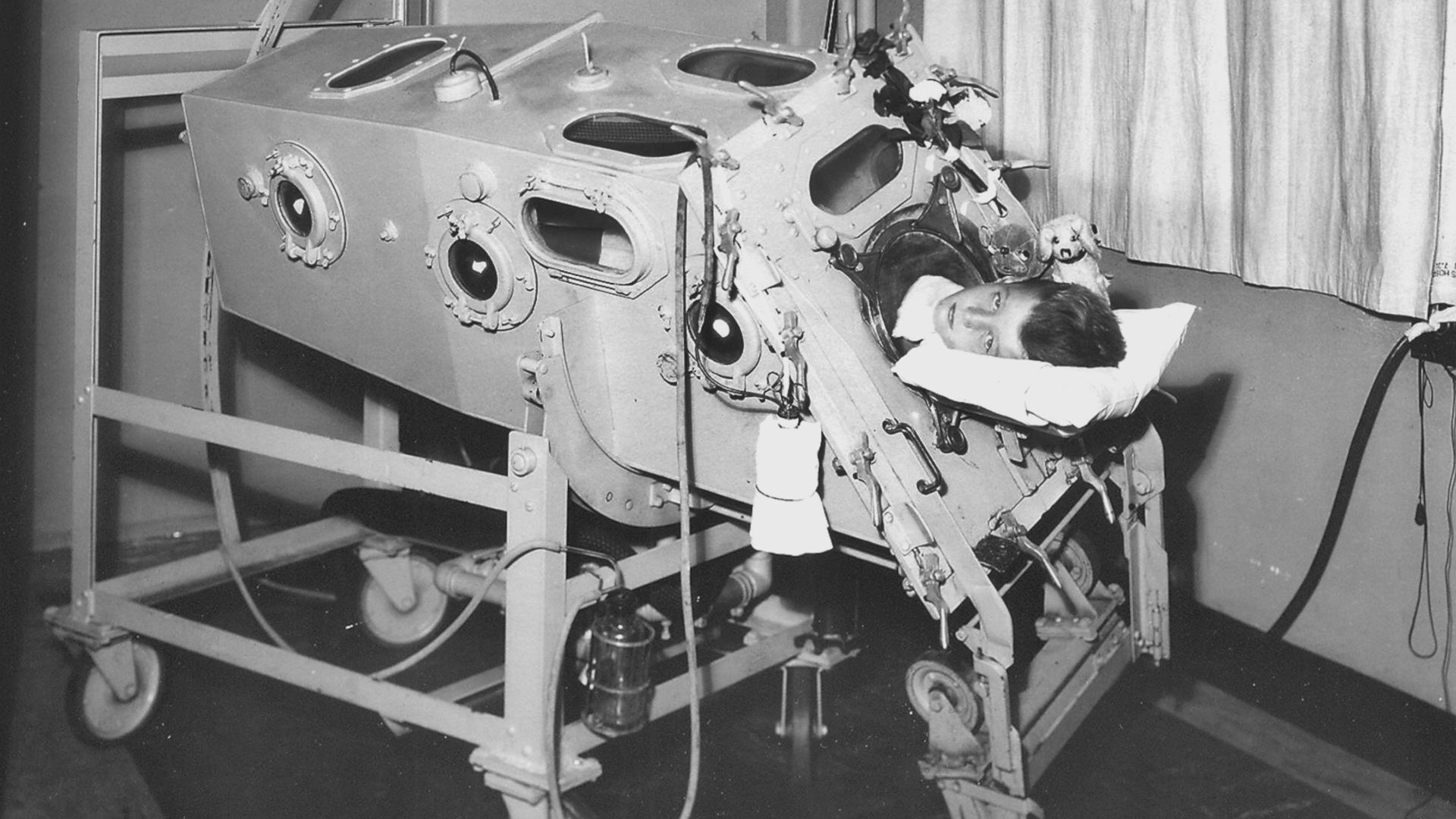
In the 1920s, Philip Drinker (right) and Louis Agassiz Shaw Jr. (not shown) developed the “iron lung” by first experimenting with anesthetized cats, initially pumping air in and out by hand using a syringe.
Visual: Harvard Medical Library collection, Center for the History of Medicine in the Francis A. Countway Library, Harvard University
Intrigued, Drinker and Shaw began to tinker further, using cats for their experiments, and showed that they could keep such paralyzed animals alive indefinitely by breathing for them.
Having established that he could keep a paralyzed cat alive for many hours, Drinker traveled to New York to tell this to his “friends in the gas company.” He asked them to give him “some money to make a man-sized machine that would be big enough” to hold him and they said “sure.”
According to his sister, Drinker took the money home and began “by asking a tinsmith to make a box big enough for a man. A closet at the school contained a number of secondhand vacuum cleaners, discarded by a company in New York state that manufactured industrial fans. With two of these cleaner motors and a generous amount of adhesive tape, Phil made a pump and hooked it to the box.” To slide a patient into the tank, they used a garage mechanic’s “creeper,” the wheeled board that slides a person under the chassis of a car.
Once they had finished experimenting on cats, and with the human-sized version complete, Drinker and Shaw moved on to experimenting on themselves: First Drinker himself went into the box, and then Louis Freni, a morgue worker at Harvard who was assisting them.
Drinker was not a physician and was unfamiliar with polio patients — he was originally thinking about resuscitating workers who had accidents. However, during the same period as he was experimenting with cats, Drinker was approached by the physician-in-chief of what was then called Children’s Hospital Boston, Kenneth D. Blackfan, to help with the care of premature infants.
Blackfan had recognized that premature infants could not regulate their temperature, which fluctuated with the ambient air, adding to their risk of death. He knew that keeping these infants at a steady temperature would help them survive. In a precursor to modern incubators, Drinker designed “conditioned nurseries” in which the air temperature could be tightly controlled, procuring fans, blowers, and other needed equipment through his relationships with various gas and electric companies.
Drinker was frequently called to make adjustments to these hothouses. On one of these visits, Blackfan and his colleague, James Gamble, mentioned that they needed help for children in the terminal stages of respiratory paralysis from polio. “With some misgivings,” Drinker went to the children’s hospital wards. He saw “a couple of these unfortunate children expire” and described it as a “harrowing experience.” His sister wrote that “he could not forget the small blue faces, the terrible gasping for air.”
And so Drinker’s plans unexpectedly shifted from the resuscitation of gassed and electrocuted workers to polio patients. He and Shaw did further testing, figuring out how to get a good seal on their tank and determining how much negative pressure to exert to suck open the lungs consistently.
They also noted that when the body was sealed into the metal tank and the pump wasn’t running, it quickly got too hot, and they needed a way to cool down the interior of the machine. They fitted a small blower to the box that would contain the body and circulated the air through a can of ice, which both dehumidified and cooled the air. For all this refinement, they used as test subjects “normal men and women, chosen at random from the laboratory personnel in the building.”
The new machine was finally ready to their satisfaction, and they tried it out on a patient on October 13, 1928. Bertha Richard was 8 years old and lived with her father, Alexander, a construction worker, and her mother, Madeline, in Waltham, Massachusetts. She had been sick for three days, developing a fever, headache, and stiffness of the neck and back.
By the time she was admitted to Children’s Hospital Boston on Friday, October 12, her left arm was weak and she had difficulty breathing. She received a spinal tap, a procedure that was used to help confirm the diagnosis of polio. Knowing Bertha had little chance of survival, the pediatrician Charles McKhann called Drinker and Shaw. They wheeled their new contraption over to the children’s hospital and left it in the little girl’s room; they turned it on next to her bed so she could get used to the sound — the early pump used was very loud.
By the afternoon of October 13, Bertha’s breathing was worse, and at 4 p.m. she was placed in the machine, mostly to test it out to make sure it was working and get her used to it. She did well. They took her out, as they felt she didn’t really need it yet. But Bertha was clearly deteriorating; first the muscles of her chest and neck had become paralyzed, and soon her diaphragm as well. By 6 a.m. she was struggling to breathe, with the telltale blue lips and fingers showing a lack of oxygen in her body.
She was put back into the machine. This time Drinker and McKhann increased the pressure setting up to negative 30 cm of water, so that with each cycle, the machine created that much negative pressure in the chamber around her body, sucking the lungs open. As the pressure fell back to zero, and then swung to a positive pressure (15 cm of water), the lungs would collapse back down, causing her to exhale. In just 15 minutes she improved dramatically. When she was able to speak, she asked for ice cream.
Drinker was so relieved he started to cry. Bertha was briefly removed from the machine and placed back in her own bed, but by 4 p.m. the same day, she was back in the respirator. She described that she could “breathe bigger” in the machine.
The residents took turns sitting with her day and night. Despite the machine, pneumonia overwhelmed her, and she died at 8 p.m. on October 19, aged just 8 years, 7 months, and 10 days. She had been in the new contraption, supported in her breathing, for 122 hours.
The experiment was nonetheless deemed a success. Before the pneumonia had set in, when the problem was just the paralysis from polio, Bertha had been comfortable and able to breathe, and even speak, when in the machine.
One of the next patients was Barrett Hoyt, a Harvard student. He was an assistant manager for the varsity ice hockey team and an undergraduate just a year away from finishing his degree when he was admitted with polio to the nearby Peter Bent Brigham Hospital on September 13, 1929. He was gasping and choking when placed into the giant tube; within a few minutes, he simply said, “I breathe.”
Barrett, described later by Drinker as having experienced a “long siege in the machine,” ultimately recovered. Drinker and Shaw published their careful observations about the construction and use of the new device in The Journal of Clinical Investigation.
Suddenly, respiratory failure was not a death sentence. Their version of a negative pressure breathing machine caught on in the medical community. Other hospitals started using the machines, and The New York Times and other papers began reporting on individual patients cared for in the new respirator and giving details on how they fared. The machine captured the public’s imagination.
The Drinker and Shaw respirator became the Drinker-Collins respiratory, often shortened to the Drinker respiratory, leaving poor Shaw out of the limelight. Referred to as a “mechanical lung” and then a “metal lung” in The New York Times, the name that ultimately stuck was the “iron lung,” a term that suddenly appeared in the U.S. on October 1, 1930, when a number of newspapers carried the Associated Press article about the “drinker respirator, commonly known as the ‘iron lung,’” that was rushed by truck from Harvard up to Maine, and “was credited with saving the life of Norman Hibbard of Bridgton.”
The monster metal device was about to become synonymous with the treatment of polio. The relationship between human and machine had been irrevocably changed.
While the iron lung was a marvel of technology, Wilson noted that caring for large patients encased within, such as Barrett Hoyt, was particularly difficult. The patient was laid out on a narrow table and slotted into the tube like a tray of pastry into the oven. At this point, with their head sticking out one end, they were fully enclosed to create the necessary seal, giving carers no easy access to the patient’s body.
It took six people to bathe a patient. The clinical team had to stop the machine, slide the person out, and move as quickly as possible to complete the task before the patient turned blue, slowly asphyxiating before their eyes. As soon as they were done, they’d shove the patient back in and start the machine again.
And then there was the noise. The pumps that powered the respirators, supplied by the Electric Blower Company, were reliable, but “their noise is a drawback in hospital work,” Drinker and Shaw noted in their first paper.
Wilson was tasked with improving on the original design, particularly the accessibility of the patients. He went down to Atlantic Avenue in Boston and bought some portholes, which were welded on and fitted with rubber collars. A nurse or doctor could pop open the portholes, stick their hands in to create a seal, and care for the patient without losing the negative pressure and the rhythmic breathing.
It was still cumbersome, but a big improvement. Later versions pillaged from the automobile industry; British models incorporated parts of the Morris Minor car, including the gasoline cap, to allow tubing in and out, and a steering wheel to rotate the iron lung (and the patient within) like a rotisserie chicken.
There was no vaccine against polio in 1928, and still little understanding of how the disease was transmitted. Cases were inevitable, and hospitals throughout the world saw a steady stream of polio patients. With the iron lung, doctors could finally do something to change the natural course of the disease. Now people who would have died from respiratory paralysis might survive.
Both inventors went on to do renowned work on industrial toxins (Philip Drinker) and compressed air illness — the bends — in deep sea diving (Louis Agassiz Shaw). But because the respirator they invented was literally a lifesaver, it was always that “damn machine,” as Drinker called it, that the two men were known for.
Barrett Hoyt, who had spent four weeks in the iron lung, and a year battling polio, graduated from Harvard a year late, in 1930. He went on to work for many years for the Liberty Mutual Insurance Company in Boston, and was even able to play golf. He died in 1972. That “damn machine” gave him 44 years more of life.
Hannah Wunsch is a critical care physician and researcher at Sunnybrook Health Sciences Center. She is a professor of anesthesiology and critical care medicine at the University of Toronto as well as a Canada Research Chair. She lives in Toronto, Ontario, and Woods Hole, Massachusetts.










And then there was a vaccine! Anyone afraid of vaccines should read this article, the book, and be grateful for wise and creative and determined researchers and doctors who give us vaccines. Polio was a nightmare. If a disease has got a shot, get it!