Virus Hunters: The Daunting Search for the Next Deadly Pathogen
Kimpese, a mid-sized town in southwestern Democratic Republic of the Congo, rings with a cheerful melody as the thumping bass of café sound systems mixes with the chatter from vendors selling bright fruits and soft fabrics. Against the background of this everyday chorus, it’s almost impossible to hear the tall man with grey-tinged stubble, standing beneath the overhang of a single-story, freshly painted building, talking to the bats overhead.
He speaks in a hybrid language of chirps and the local Kikongo dialect, cooing toward the outdoor rafters as mothers steer their children around the eccentric visitor and into the facility — the local health center — stealing curious but wary glances. The animals respond with a flurry of clicks and squeaks. “They’re happy to see us,” he says, laughing softly.

The irony of potentially disease-carrying bats lingering in the rafters of the local health center is not lost on Mulembakani.
Visual: Jeffrey Marlow/Undark
The bat-whisperer is Dr. Prime Mulembakani, and along with a couple of colleagues from the DRC’s Institut National de Recherche Biomédicale (INRB) in Kinshasa, he is in Kimpese to test bats for potentially dangerous viruses as part of a U.S. Agency for International Development effort called Predict. Launched in 2009 as part of USAID’s Emerging Pandemic Threats program, the Predict project was renewed in 2014 with a grant of $100 million over five years to develop a sprawling, searchable database of the zoonotic pathogens behind emerging pandemic threats in countries around the world.
If scientists can detail the places where lethal viruses simmer in wait, the thinking goes, they can head off a swelling pandemic and better manage outbreaks while they are still small and local. Researchers and other outbreak responders could consult this database to begin mapping the source of an emerging disease, for example, and quickly get to work on minimizing transmission and developing potential new vaccines that could save countless lives.
“We have a job to do,” Mulembakani explains. “We also have the opportunity to be in contact, in close contact, with people who are on the front line — the communities who are really at risk for a virus spillover from animals into people.” The irony of potentially disease-carrying bats hanging from the rafters of the local health center is not lost on Mulembakani, an epidemiologist by training, and he pauses for emphasis: “We need to stop these events from getting out of control.”
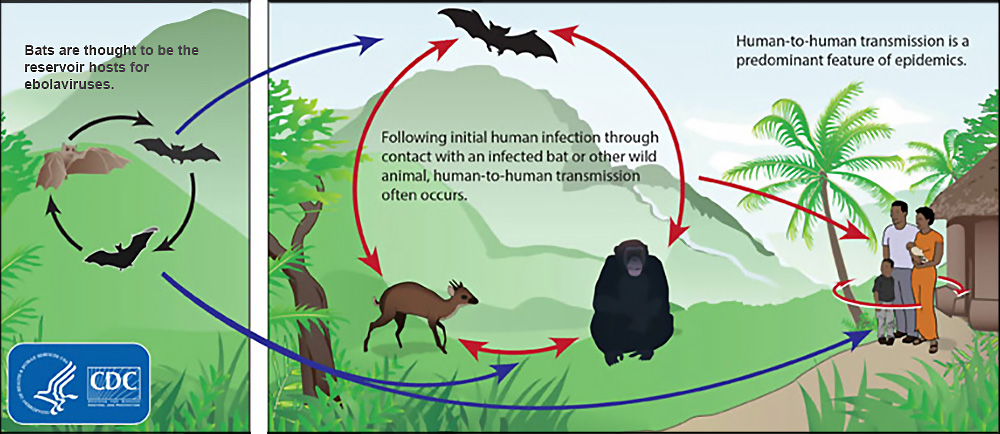
It’s an ambitious idea, and the stakes are high. The infamous 2014 Ebola outbreak, after all, likely began when a toddler came in contact with bat droppings while playing near a tree in rural Guinea. The virus went on to kill more than 11,000 people over the next two years, orphaning as many as 30,000 children and damaging affected West African economies. Similarly, the 2009 H1N1 “swine flu” pandemic that caused the deaths of nearly a quarter million people may have started when a five-year-old became infected in a mountain village 120 miles east of Mexico City. HIV/AIDS — the most devastating spillover of the last century — is credited with an estimated 35 million victims after the virus jumped from primates to humans in the 1920s.
And just two weeks ago, the World Health Organization declared another Ebola outbreak — this time in the northern part of the DRC.
Experts fear that spillover events like these are going to become more common in coming years. The increasing rate of human encroachment into animal habitats is removing the old buffers, making contact with potentially infected animals more common. And the math is simple: More points of contact facilitate more spillovers, and more transmission of animal pathogens into the human population. Add to that the globalized transportation network, in which billions of people (or potential hosts, from a virus’s perspective) are just a plane ride away, and the risks appear to grow at an incendiary rate.
That may sound daunting, but the era of “big data” virology is on the horizon, and it is now helping to drive embryonic efforts like the one here in Kimpese. “Right now, for example, we try to separately develop vaccines for SARS and MERS, which are both coronaviruses,” says Eddy Rubin, chief science officer at Metabiota, a San Francisco company that oversees several of Predict’s operations, including the one in the DRC. “If we had 10,000 coronavirus sequences, it’s likely we would identify common features, so that instead of making vaccines against individual viral species, we could be making vaccines against full viral families.”
This would, of course, take a lot more commitment and a lot more money than is currently on the table —something similar to the billions of dollars underwriting large-scale efforts like the Human Genome Project, Rubin says. A similarly ambitious global virome project, he adds, “could expect to have have even greater payback.”
That sort of support, however, seems unlikely to materialize anytime soon — particularly given drastic cuts to foreign aid and development programs, including USAID, under the latest budget proposed by U.S. President Donald J. Trump. As it currently stands, the Trump proposal would slash the budgets of the State Department and USAID by a little over $17 billion — a 31 percent cut.
For now, then, Mulembakani and other Predict team members around the globe are charging ahead with what they have — though even their strongest advocates admit the mission faces formidable obstacles. Money aside, there are more than a billion viruses in a pinch of soil, and scientists have only just begun charting the landscape of viral diversity. Most viruses have no noticeable impact on our health. Some are probably helpful, and only a tiny fraction cause disease. That makes figuring out where known viral threats lurk, and determining which new viruses are dangerous, tasks with very low odds of success. “I think statistically speaking,” Mulembakani says, “it’s a challenge to say that we will find something that will have a great impact on many people’s lives.”
And yet, having lived through past pandemics, Mulembakani also believes that even a flicker of a chance to control an onrushing infection is reason enough for him and his dogged colleagues to push on, visiting obscure villages, unsanitary markets, and overcrowded hospitals, testing for the signature of worrisome pathogens. “The advances in medical biology, laboratory techniques, and epidemiology,” Mulembakani says, “make it possible to explore the world of viruses with pandemic potential and make the world safer.”
This sort of optimism in the face of what is, by almost any measure, a long-shot mission punctuated by daily setbacks, political and infrastructure roadblocks, and other frustrations, is a crucial characteristic for the virus hunters. For them, the notion of doing nothing to prevent the next spillover of a deadly virus from animals to humans is simply not an option.

The day before he arrived in Kimpese, Mulembakani and his team set forth to Wene — a tiny a cluster of huts without running water or electricity — for one of their bat sampling visits.
It was onion-harvesting season in the fertile valley at the foot of the Crystal Mountains, and the roads were noisy with overloaded motorcycles carrying the produce to larger market towns, kicking up plumes of ocher dust. Through a network of informants, the Predict team had heard about a nearby cave where villagers gathered guano — the nutrient-rich bat droppings that enabled the bountiful onion harvest — and they were eager to collect some samples.
A long-time guano harvester, Nzuzi Yalungana, agreed to lead the INRB researchers — Mulembakani, Guy Midingi, and Ipos Lukusa — to the site as the sun began to set. On the trek to the cave, Yalungana passed the time by recalling earlier visits. “We would go in groups of three,” he explained, “with headlamps, buckets, and spades, and we filled bags.” The caves were not welcoming he warned: “It was dusty in there, not good to breathe.”
To reach the cave, the team climbed a steep hill through tall golden grass, along a ridge marked by gangly trees, and down a steep slope to the cave’s gaping mouth. Midingi and Lukusa suited up — wearing head-to-toe Tyvek, a full-face splashguard, and three pairs of latex gloves — and strung a net between two bamboo poles across the cave’s entrance. These “mist nets” are remarkably effective at immobilizing bats — so effective that many companies require a permit for purchase to prevent villagers from decimating bat and bird populations.
As night set in, the bat migration started slowly, but soon the net rippled outward, followed by a faint patter like the first tentative raindrops of a coming storm. Yalungana watched, expressing some bemusement at all the protective gear. “We are not concerned,” he says. “We hear that these scientists are looking for microbes that can give diseases. We’ve never seen it, we have no proof, and it’s never been a problem.”
But Yalungana’s nonchalance — and the unbuffered contact between people and wild animals it engenders — is the basic narrative of spillover stories. Scientists fear that such casual contact offers an ideal opportunity for viruses to jump into humans. But the first question is whether the Wene cave is home to pathogenic viruses. If so, it may also offer some clues as to how viruses move through animal populations.

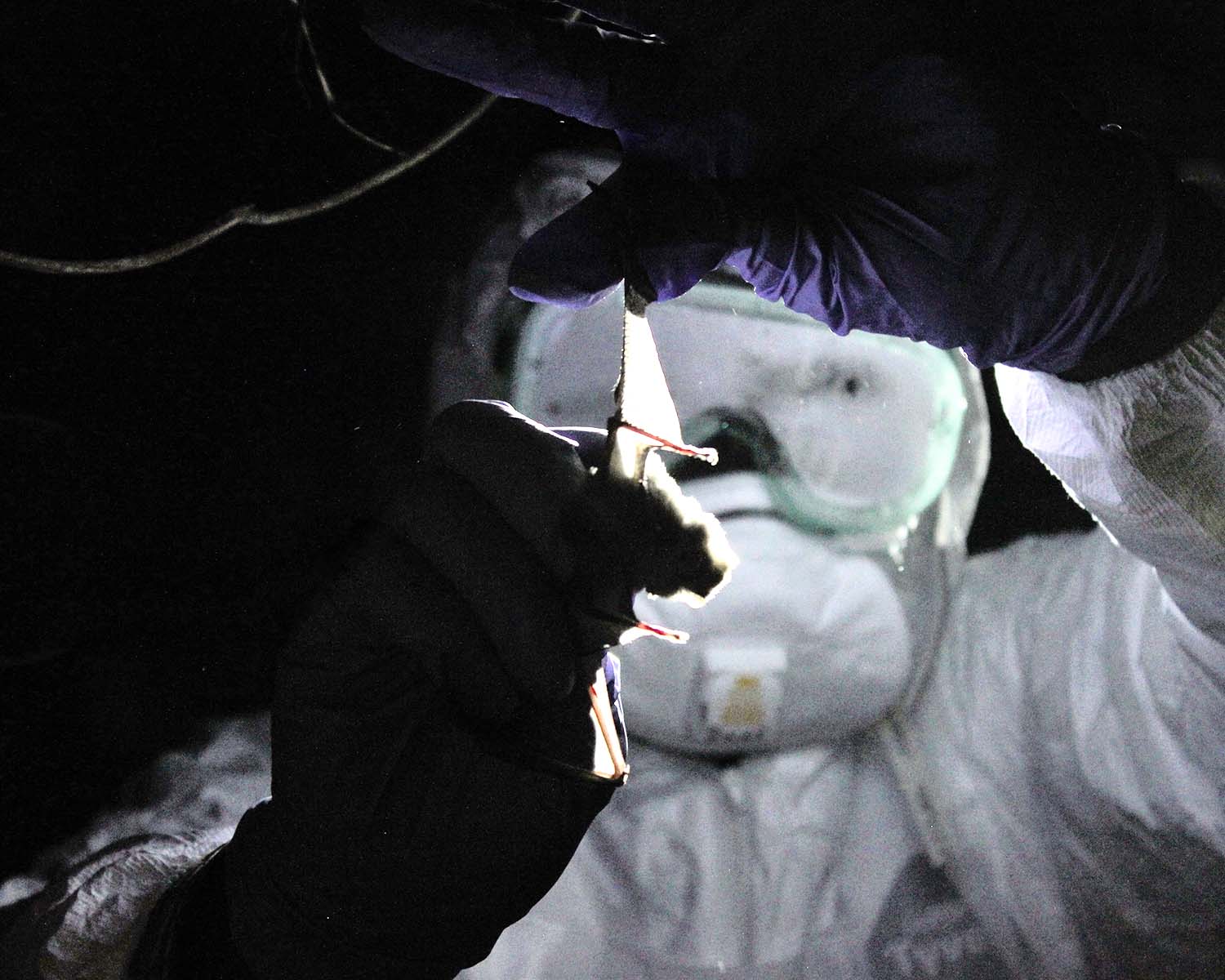

After an hour — and after trapping 13 bats, carefully sealing each into a white canvas bag — Mulembakani gives the signal to wrap up the operation. They start the downhill climb to the village, the stars and crescent moon lighting the way back to the cooking fires of Wene.
In Kimpese the next morning, Midingi and Lukusa prepare to take samples, arranging sterile tubes, chemical bottles, and pipettes on a white tiled lab bench outside the health center.
At this point, Predict’s protocol takes effect, a 96-page document that standardizes the analytical pipeline across all of the project’s research teams. Most of these globally distributed researchers work on viral surveillance projects like the bat sampling in Wene. But there are other programs, such as those looking at the ways human behavior influences spillover events, or those seeking to build mathematical models of how viruses spread.
With such a sprawling operation, where research teams often have different levels of training and face a range of logistical obstacles, it’s important to be consistent — only then are results directly comparable and globally applicable.
Mulembakani played a key role in shaping the protocol, and he speaks about it reverently. “We were the first country to receive approval from Predict headquarters for the protocol,” he says, beaming like a proud father. “Each country needs to adapt it to their area, but not much should change because we want it to be standardized.”
It’s easy to lose sight of proper scientific technique in chaotic environments: Shortcuts are very tempting when potentially pandemic-starting bats are fluttering around your head, or when supplies are held up in customs. The protocol is a bulwark against panic, impulse, and even boredom. It’s a rulebook for repetition and reliability, and the bat sampling provides a visible example of how carefully the DRC team adheres to its guidelines.
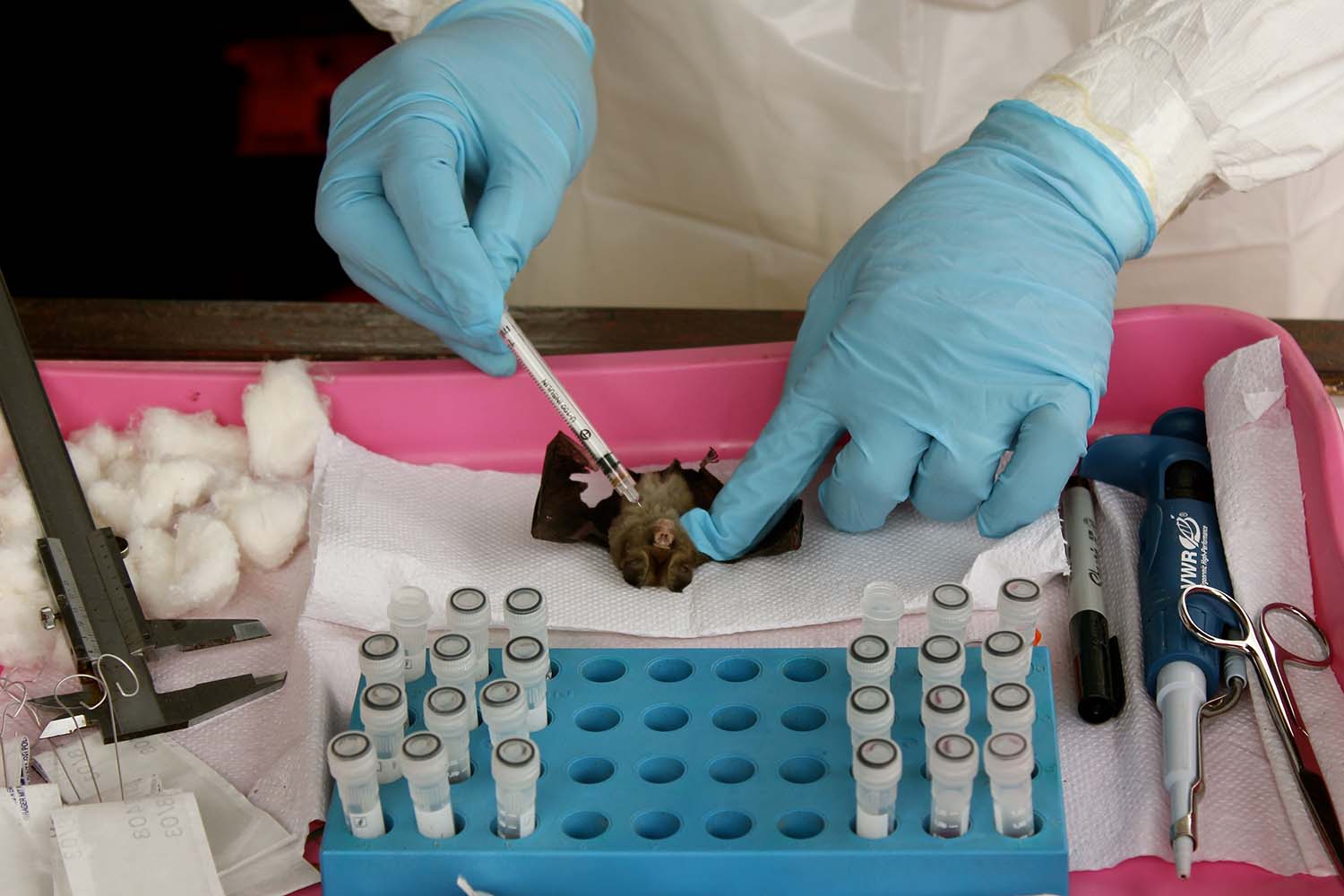
Over several hours, Midingi and Lukusa work their way through the bats that were caught in the mist net, using sterile cotton swabs to collect saliva, feces, and blood samples — the fluids that are most likely to transmit viruses. It’s an almost rhythmic exercise: swirl, swab, cut, prick, twist, repeat. As soon as the samples are collected, viral particles and their genetic material start to degrade, and it’s a race against time to keep valuable data from slipping away. Samples are mixed with solutions that stop metabolism and are immediately placed in a vat of liquid nitrogen: Plunged to -321 degrees Fahrenheit, degradation processes slow down dramatically, allowing researchers to handle the samples back in the lab days or weeks later.
By the time the last tube is sealed, seven of the bats have died, and Mulembakani is disturbed by the toll their sampling effort has taken. Nursing the lethargic survivors back to health, he feeds the bats concentrated sugar water through a dropper, and they stagger drunkenly across the table as they regain strength.
“There could be something already weakening them,” Mulembakani says of the alarming death rate, pointing out a parasitic growth on one bat’s stomach, and an oozing wound on another’s shoulder.
“Usually maybe one or two die — we’ve never seen this before.”


The team’s next stop is Kinshasa, the DRC’s capital and largest city, with a population of more than 11 million. At the edge of the crowded road, children gather at speed bumps to sell bowls of writhing insects to passing cars. They find Mulembakani to be an enthusiastic customer: He loves to eat grasshoppers, termites, and grubs, but crickets are his favorite. He orders a full bag, and places it at his feet in the back seat of the car.
Born in 1961 in Bandundu Ville, a city in western DRC, Mulembakani used to be one of those kids, running around catching insects to sell at the weekly market. By night, he would stare at the stars for hours, tracking his favorite constellation — the “strong, tenacious” Orion — across the sky. After moving to Belgium and then back to the DRC at age 8 — his father was a sought-after soccer coach at the time — Mulembakani noticed the jarring economic fault lines that separated western Europe from central Africa.
The root of it all, he came to believe, was poor health: If people couldn’t recover from a trivial injury, or if they died from preventable diseases, then how could they be expected to live productive lives?
Mulembakani enrolled in medical school, and near the end of his studies he was faced with a tough choice. His father had lined up a well-paid job with a Belgian company in Kinshasa — indeed, he had already promised the CEO that his son would take the position — but Mulembakani hesitated. While mulling it over, he found himself staring at posters in the university’s halls about monkey pox research, complete with photos of debilitated children: “You could see what this disease did to people. I was touched to see how people were suffering from diseases we weren’t even studying — these were the problems we needed to be solving, not hypertension and arthritis.”
Instead of accepting the comfortable position in Kinshasa, Mulembakani moved to Genge, a small village more than a thousand miles away with no running water or electricity. “It was a very big fight between me and my father, but other people needed my help more.”
Over the next 20 years, Mulembakani blossomed into a celebrity doctor (“People would come from 200 kilometers away to see me,”), got co-opted by the rebel army during the civil war (“We took ammunition to the front lines and brought wounded soldiers back to treat them,”), and earned a Master’s degree in North Carolina (“I only had money for two semesters, but I saved, ate little, and made it three”).
His wide-ranging experience made Mulembakani well suited for his role at the helm of the DRC’s Predict node. He’s breezy and relatable with villagers, rigorous and exacting with scientific colleagues, and charming and upbeat with ministers and international donors. Through it all, his singular laugh lightens even the most worrisome moments. It starts with a chuckle — a couple of octaves higher than expected — and escalates to a rasping hilarity, with staccato aftershocks as the memory of the joke lingers.
As the Land Rover enters the bustle and buzz of Kinshasa, this laugh fills the car: The crickets have gotten loose, and Midingi is frantically sweeping them back into the bag. A few lucky escapees make a run for it as Mulembakani gets out at the INRB. The insects vanish as the men open the car doors to unload their gear and carry it to the green one-story virology building, moving quickly past the resident population of bleating goats in the INRB’s central courtyard.

According to the protocol, the first order of business is ensuring that the precious samples take the next step in the “cold chain,” the consistent maintenance of sub-freezing temperatures to protect the viruses from degrading beyond recognition. Midingi and Lukusa work together to haul the large vat of liquid nitrogen to the lab, where two lab technicians hurriedly clear a path to the hulking -112 degree Fahrenheit freezer. Lukusa opens the door and vapor cascades out, swirling at his feet as he safely stores the samples in a metal rack.
“Welcome back,” says Maria Makuwa, the team’s resident virologist. “I need your sample report, before you forget.”
While the sampling team was collecting bats, Makuwa was attending a logistics meeting where organizers shared new instructions for tax exemption requests and importation procedures. Operating a biological research lab is a complex, multidimensional puzzle in which funding, supplies, equipment, labor, and analysis need to fit together. Working in the DRC, where corruption can complicate even the simplest action, requires an additional level of logistical wizardry. At a wiry, tenacious 65, Makuwa is up to the task. She was born and raised in former Czechoslovakia — evidenced by her no-nonsense directness — and has developed her street smarts after nearly four decades of public health experience in central Africa.
“There used to be a lot of fraud,” she says. “Once, we had permission to buy 100 kilos of supplies, and when the final paperwork comes, it’s three times more expensive than they had said.” To help ease the burden on country managers, Metabiota has started to order supplies centrally through its headquarters in San Francisco. This arrangement allows people like Makuwa to spend more time on their scientific work, but the distance can lead to its own set of problems.

The INRB in Kinshasa. Working in the DRC, where corruption can complicate even the simplest action, requires a high level of logistical wizardry.
Visual: Jeffrey Marlow/Undark
The Kinshasa Predict center occasionally receives documents intended for the Sierra Leone or Cameroon teams, and the insistence on original order forms — not copies — delays shipments of critical supplies. “It’s starting to be clearer, but in the beginning it was,” she rolls her eyes, “very difficult.” And that’s just to get the supplies to the lab; Once they’ve arrived, there are often more surprises in store. Makuwa once tested 50 different DNA extraction kits — pricy supplies that use proprietary solutions developed by biotech companies — and found that just four of them worked.
Over time, the protocol has helped to smooth out this uncertainty, incorporating only the most effective reagents and well-established methods into the team’s workflow. It goes like this: A tube the size of a pen cap, containing a sample and a preservative liquid, is taken from the freezer and thawed, gradually, on ice. “We first look at the ones from oral and rectal swabs,” says Lukusa, “since that will tell us if the animal is shedding the virus and is especially dangerous.”
During the next couple of hours, the tubes are shaken, spun, heated, and cooled — all part of the process to extract RNA, the molecule that animals and viruses alike use to encode proteins. These steps are intended to crack open the biological material in the sample: from the comparatively massive eukaryotic cells of bat tissue, to simpler gut microbiota, to tiny viral particles.
A typical virus is about a million times smaller in volume than a bat blood cell, so one challenge is sifting the evidence of viral genetic material from the background of more abundant cells with larger genomes. To help with this, the team uses reverse transcriptase polymerase chain reaction (RT-PCR), converting RNA into complementary DNA and replicating it over and over until the viral genetic signal rises above the bacterial and eukaryotic noise. Still, there are limits — largely financial — to what they are testing for and, for all his enthusiasm, Mulembakani chaffs at the restrictions.
“At this point, we are fishing,” says Mulembakani, “looking for five different viral families.”
Most of the major viruses that can emerge as pandemics come from these families. The suspects — Coronaviridae, Paramyxoviridae, Filoviridae, Flaviviridae, and Orthomyxoviridae — are families that include well known killers like Dengue, Ebola, SARS, Marburg, and the flu. The selection of these five viral families was a difficult decision for the Predict project. The team sought a balance between comprehensiveness — these five families represent just four percent of total viral diversity — and the cost of a meticulous analysis. In the end, examining all 122 known viral families was deemed to be less effective than thorough analysis of the known worst threats.
To target deadly zoonotic infections, the five-family short-list is a good start, according to Nathan Yozwiak, an associate director of viral genomics at the Broad Institute, although he might add a couple of additional groups. “These are the major families of RNA virus that cause acute disease,” he noted in an interview, “but it is certainly not an exhaustive list. The one big thing that’s missing are the arenaviruses, RNA viruses that include things like Lassa, for example. DNA viruses like herpesviruses can cause a lot of diseases too.”
But even with this compact list, a comprehensive analysis is time-consuming and costly: At $43.40 per sample (not including scientist salaries), the thousands of analyses can add up. After the RT-PCR process, samples are dyed and a few microliters from each tube are injected into different lanes of a gel made with agarose (a polymer derived from seaweed). When an electric current is applied along the length of the gel, the DNA starts to migrate through the agarose thicket. Like children running through a crowd, smaller molecules of DNA move faster, and the size distribution of DNA fragments can be determined. If a distinct, bright band shows up at just the right spot — something that happens about 10 percent of the time, according to Mulembakani — that’s the likely sign of a target virus.
But even then, further analysis is needed to assess the risk. The Coronaviridae family, for example, includes everything from the common cold to SARS, which incited global panic in the early 2000s. To find out whether the sample is benign or deadly, the material is shipped to Germany, where the tests are run and the genetic sequence of the amplified portion is read, annotated, and emailed back to the INRB. The team then sends the results to the U.S. for confirmation.
Which means that every such set of results is examined with some curiosity — and some anxiety.


In the fall of 2012, the Predict team in Kinshasa heard back from international colleagues and knew that a summertime mystery had been solved. Over the previous several months, four bonobos from the nearby Lola Bonobo Sanctuary had died under mysterious circumstances, raising fears of an epidemic among the apes that could spill over into the sprawling Kinshasa megalopolis.
The results pointed to encephalomyocarditis virus, which preys on the heart and kills quickly. “[They] have the symptoms in the morning,” says Claudine Andre, the sanctuary’s founder, “and in the night time they are dead.” The high mortality among bonobos — one of humanity’s closest relatives — was particularly concerning, since a virus’s virulence is often similar within closely related species. Ever since this episode, the Predict team has been working with Andre and her staff to keep an eye out for suspicious viruses among the bonobos.
Lola functions as a halfway house for infant bonobos rescued from the illegal wildlife trade. Plucked from the black market in Kinshasa or Kisangani, they’re taken to the sanctuary, nursed back to health, and socialized into groups. They romp around large enclosures during the day and spend their nights indoors. If all goes well, they are reintroduced to their native habitat in central DRC.
The sanctuary occupies 75 acres of forest on what used to be the outskirts of the capital, nestled beneath a ring of cliffs. Now, Andre feels besieged. A dam on the valley floor, built by a Chinese company, is creating pools of stagnant water that incubate tiger mosquitoes just outside Lola’s gates. From the massive industrial chicken farm atop the bluffs, “feces come with the rain down the mountain and into the enclosure, and their cows graze at the spring that feeds our sanctuary,” Andre complains. “We are surrounded by problems.”

Caring for a sick bonobo at the sanctuary. The deaths of four of the animals in 2012 was traced to the encephalomyocarditis virus.
Visual: Maria Makuwa/USAID
This ecological mash-up is another situation that scientists recognize for spillover potential. It brings insect vectors, antibiotic-treated livestock, semi-wild animals from many different forests, and a diverse, transient group of people together, generating a potent brew of genes, mixed among species. This makes the refuge a useful bellwether for Predict, and a petri dish microcosm of the region. “Chimps, monkeys — you can link a lot of viral diseases to them,” says Makuwa. “But it’s rare to find a virus in bonobos,” making their detection all the more concerning and constant vigilance all the more necessary.
“As this area changes, very strange things will come out of the forest.”
As a student in Soviet Bratislava, Makuwa says she was fascinated by the electron microscope, a massive instrument that revealed startling insights through images: stringy cell membranes and steampunk viral injection systems. “They told me it was not for the girls,” she recalls, “that it was only for the boys because you need to know physics and optics…I showed them that I could be completely self-sufficient: prepare my samples, use the microtome, make the examination. I was the only girl allowed to work there.”
Actually seeing the miniscule entities that can so swiftly dispatch animals billions of times their size was a revelation. “These days, to prove there is a virus, you don’t need to actually see it,” she explains. It’s more efficient, she acknowledges, but she sometimes thinks that a reliance on genetics can have a downside: “We lose some of the immediacy and the personal impact.”
It was in Bratislava that Makuwa met her future husband, a native of the former Katanga province in the southern DRC. They moved to Kinshasa in 1978 and she was charmed by the vibrancy of the city. “Back then, I knew Kinshasa by night, I was very young,” she says wistfully. “Every night, we were outside, to drink, to dance, every evening somewhere.”
But if Kinshasa has become less charmingly urban, Makuwa remains entranced by the nanoscale world of viruses, scanning the scientific literature each morning for relevant studies and new data. With each scientific paper, researchers are discovering just how chillingly impressive viruses can be: They’re the freshly sharpened point of an evolutionary arms race with hosts like ourselves. For example, the diminutive Ebola virus has just seven genes, and yet is quickly able to dispatch a human host, 19,000-genes strong. Its genius lies in provoking a self-destructive response, disarming the body’s immune system to gain access to vital organs.
Such unnerving efficiency, combined with rapid evolution and the uncertainty of a host’s physiological response, can make viruses seem almost fundamentally unknowable. Despite the reams of genetic sequence collected by Predict, and the project’s suggestive title, the data are not predictive of risk: Knowing the sequence of a new virus does not necessarily tell scientists how dangerous it will be. “You can make inferences, ideally, about what role each gene segment might have,” explains the Broad Institute’s Yozwiak, “and that would be useful, but making larger leaps about its phenotype in people or any other reservoir species, that’s a lot harder to do.”
But it’s also an essential first step, and as more sequences stream in from the mountains of Ethiopia, or the rice fields of Vietnam, and the caves, forests, and even the cities of the DRC, the models will grow stronger.
Kingasani is a bustling, chaotic neighborhood on Kinshasa’s eastern margin — notorious among INRB staff for its drug trade and red light district. It’s also the first stop for many new urbanites, the droves of villagers flocking to the capital in hopes of a better life. These migrants frequently bring illnesses with them from the bush, making Kingasani a hot zone worth watching, as potential zoonoses from tens of thousands of square miles are concentrated in the slum.
The Kingasani Hospital is sequestered from the putrid, pitted roads by a 10-foot tall concrete wall. A $4 fee buys you a medical consultation. It’s a good rate, and as Mulembakani and his INRB entourage are waved through to meet with the nuns who operate the facility, dozens of ailing patients lie on mats outside the gates, begging for the copay, on the threshold between the squalor outside and the orderly hospital wards.


Hypothetically, this is the kind of place where a guano collector like Yalungana, from a tiny village like Wene, might end up if he scraped his leg in the bat cave and developed a persistent cough or an untreatable fever. He could spread this illness to other patients, who may be discharged before symptoms develop. As the number of victims accelerated, health officials would be scrambling to figure out what the precise infective agent was, where it came from, how it was transmitted from person to person, and how best to sever the chain. The time needed for such assessment means that critical days would be lost in heading off a fast-spreading epidemic.
The Predict team hopes that their information will give outbreak responders a valuable head start in such a case. Once detected, the unknown virus can be quickly sequenced and cross-referenced with the database that Mulembakani and his colleagues around the world have been building. At Kingasani Hospital, he is looking to cast an even wider net by sampling human patients for viruses (a process that has been approved by both the local and U.S. Institutional Review Board, which assesses any experiments on humans for both efficacy and ethics).
It’s something of a philosophical shift for the INRB team, moving from a single-minded focus on surveillance to early detection — looking for viruses post-spillover in a way that can inform the public health response. But it’s also an idea with obvious promise: After a 10-minute meeting with the nuns running the health facility, Mulembakani has successfully convinced the hospital to start sampling their patients.
“This is a big step for us,” Mulembakani says as the hospital’s gate clangs shut. “If we find new pathogens from [sick patients,] our team can conceive new assays.”
He hesitates for a moment, realizing that some additional training may be in order: “I just hope they use the protocol correctly.”
Early evening fires across Kinshasa’s outskirts thicken the air with the drift of wood smoke. In the hazy twilight, over a dark bottle of beer, Mulembakani decompresses at an outdoor cafe, shifting from exuberant salesman to slightly depressed realist.
Over the last seven years, his team has sampled thousands of animals at hundreds of sites, across some of the most diverse ecosystems in the world. They’ve begun building a database of virus distribution and have started working with the national health ministry. And yet, the astronomical number and fractal diversity of viruses could be an insurmountable gap, and the goal of finding viral killers before they begin their rampage may be little more than an ambitious human hope that wilts under nature’s reality.

In the DRC, where urgent and tangible problems pervade life on a daily basis, this level of abstraction can be hard to accept. More resources should be directed to daily demands of security, food supply, and economic growth, critics might argue, rather than a quixotic quest to catalog microscopic organisms. To Mulembakani, however, pandemics are nearly unmatched in their ability to destabilize society, and preparation is the key to prevention. As he sees it, he may not be running around putting out fires — but he’s developing a theory of the flame, mapping its contours and studying its ravenous tendencies in order to develop an effective response.
“We don’t know one one-hundredth of one percent of nature,” Mulembakani says with a sigh. “There is just so much out there. But we have a job to do.” He takes a sip of beer and shifts his gaze skyward, easily identifying his favorite constellation, Orion, the unparalleled hunter. “Maybe I will not learn something interesting,” he says, “but I might.” And that something could be everything, a discovery to prevent a pandemic and fulfill the promise he made to his country decades ago.
“I don’t really know why I care so much, but I can’t stand by. I will give everything I have to help people who suffer.” Mulembakani sits back, distractedly swatting flies from the table. The conversation shifts and, soon enough, his signature laugh is back, carrying into the gathering dark.
Jeffrey Marlow is geobiologist, writer, and post-doctoral fellow with Harvard University’s Department of Organismic and Evolutionary Biology. His journalistic work has been published by The New York Times, Wired, and Discover magazine, among other outlets.









Comments are automatically closed one year after article publication. Archived comments are below.
Fascinating! If only I could have been a scientist like Mulembakani! I’m grateful for men and women out in the field doing this kind of work. I hope we have many more young scientist studying to carry on the work.