When Addiction Treatment Fails, Can a Brain Implant Succeed?
On July 12, 2015, Elena Daly was packing for a family vacation when she walked into her 16-year-old son’s room and found him unconscious. Her son, Max, had overdosed on opioids, aspirated vomit, and fallen into a coma.
By that point, Max had struggled with addiction for about three years. He had tried medication, therapy, and residential treatment programs in France, where the family lives, as well as in the United States and the United Kingdom. In fact, his July relapse occurred just days after returning home from a six-month stint in an in-patient rehab program. The coma lasted three days and worsened a pre-existing movement disorder to a degree where Max was unable to attend high school. “I couldn’t hold a pen without throwing it across the room or hold a cup of coffee without spilling it on myself,” he recently recalled.
Max’s struggles with opioid use are not unusual: An estimated 40 to 60 percent of people who have an addiction experience relapse after treatment.
Some researchers have suggested that a substantial portion of those who relapse suffer from what might be considered a “treatment-resistant” form of the disorder, though that condition is not formally recognized as a medical diagnosis.
In recent years, scientists have explored treating these intractable cases of opioid dependence with deep brain stimulation, an intervention that entails surgically implanting an electrode into a precisely determined region of the brain, where it delivers regular pulses to control problematic electric signals. The surgery has proven effective for neurological conditions such as Parkinson’s disease and essential tremor, a disorder that can cause a person’s limbs, head, trunk, and voice to quake.
But for researchers attempting to study its efficacy for addiction, the procedure’s invasiveness and cost — typically in the hundreds of thousands of dollars — have raised steep hurdles. Work in the field has largely been limited to one-off treatments and small studies with one or a few participants, making it tough to ascertain how many people globally have received the treatment or how successful it has been for them.
“You’re in an entirely different world of claim-making and what it means to establish a scientific fact at that point,” said anthropologist Danielle Carr of the University of California, Los Angeles, who argues that such minuscule studies are limited in their value to the broader population. Carr is among a chorus of researchers who have questioned the wisdom of investing funding and effort into an unproven intervention that, even if successful, likely could not be deployed at scales large enough to curb an opioid crisis that claims more than 100,000 lives each year in the U.S. alone.
For researchers attempting to study the efficacy of DBS for addiction, the procedure’s invasiveness and cost — typically in the hundreds of thousands of dollars — have raised steep hurdles.
For patients like Max Daly, however, there is a different calculus to consider — one in which the procedure’s costs and health risks can seem small relative to the burden of a debilitating substance use disorder. In 2019, Elena was in California to teach a course at Stanford Law School when she heard from doctors who suggested that Max could receive the implant as part of a procedure to help his movement disorder. That year, clinicians at Stanford University implanted three electrodes in Max’s brain: two for his movement disorder and one for addiction. He says that his opioid cravings have subsided since the procedure, although he has once relapsed to using methamphetamine. His movement disorder is barely noticeable; his hands are steady when he tugs his shirt collar to his shoulder, revealing the bulky battery that powers the implants.
Clinicians pursuing deep brain stimulation as a treatment for opioid use disorder point to success stories like Max’s as arguments for further expanding research in the area. But in a budding line of research where every patient is, in essence, an experiment unto themselves, the path from the fringes of addiction treatment to the mainstream remains uncertain.
Addiction’s grip on the brain is complicated and often unique to the individual experiencing the disease. Its effects are mediated in part by the neurotransmitter dopamine, which affects a person’s motivation, reward-seeking, decision making, and other behaviors. For many people who end up abusing drugs, the neural pathways that mediate these behaviors seem to converge at a pea-sized region near the front and bottom of the brain called the nucleus accumbens. “This is really the part of the brain where thoughts turn into movement and where the decision, for instance, to go and seek out and take drugs is initiated,” said neurobiologist Jasper Heinsbroek of the University of Alabama at Birmingham. “It’s also where extended exposure to drug abuse leads to a lot of changes in the functioning of the neurons.”
In the early 2000s, neurosurgeons in China — encouraged by rodent studies — began experimenting with removing the nucleus accumbens in human patients to alleviate severe addictions. Researchers estimate that approximately a thousand patients at about 20 hospitals received the surgery in 2004 alone. But China’s Ministry of Health put a stop to the research in 2004, amid questions about the procedure’s safety, long-term outcomes, and possible overuse.
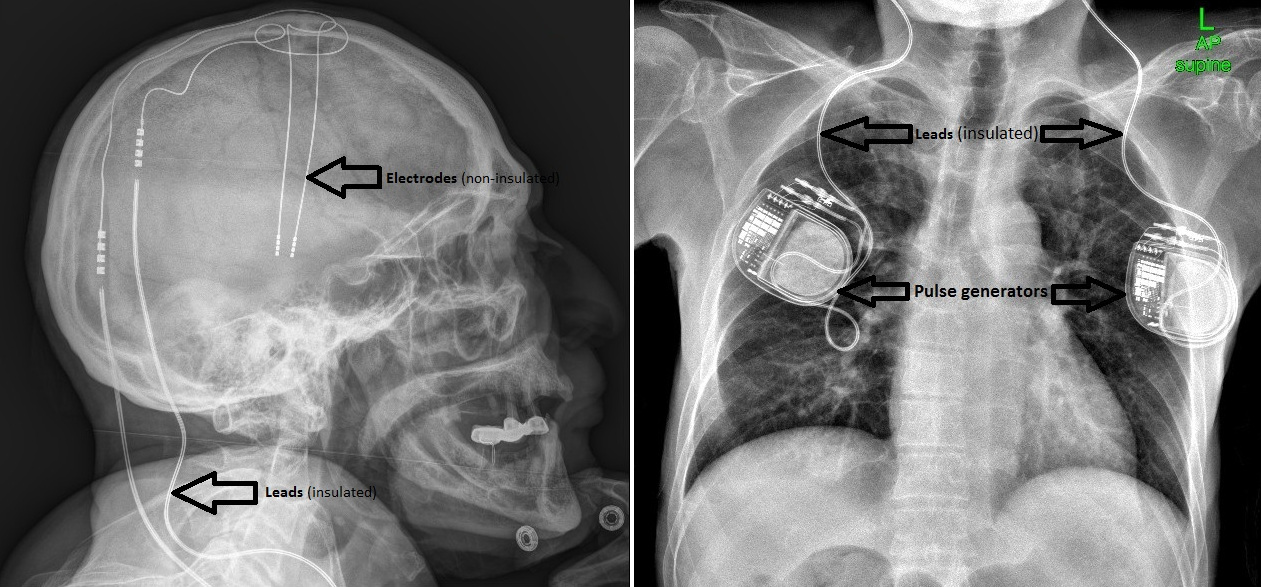
Meanwhile, a new technology was emerging that would allow scientists to alter the functioning of brain regions without removing them. In clinical trials dating back to the 1980s, DBS targeting the thalamus, the subthalamic nucleus, and other regions embedded beneath the cerebral cortex — in the so-called deep brain — have shown an ability to provide speedy and substantial relief from a number of movement disorders. Alik Widge, a psychiatrist at the University of Minnesota, recalls watching a patient being treated for essential tremor: “The patient started crying because suddenly, one second this severe, disabling symptom is there, the next second it’s gone,” he said. “It’s like faith healer, cast aside your crutches and walk kind of stuff.”
The Food and Drug Administration has approved DBS for a range of movement disorders, including essential tremor and Parkinson’s disease, and created a humanitarian device exemption that allows the procedure to be used for treatment-resistant obsessive-compulsive disorder. Over the last few decades, researchers such as neurosurgeon Casey Halpern of the University of Pennsylvania, who led Max’s 2019 surgery, have sought to deploy DBS to treat a broader range of mental health conditions that appear to be rooted in the brain’s reward processing mechanisms, including opioid use disorder.
So far, the results have been mixed.
In the late 2010s, two randomized studies of DBS for treatment-resistant depression, enrolling a total of more than a hundred participants, found no significant differences in health outcomes between intervention and control groups. Both studies were terminated earlier than planned due to a lack of convincing evidence. In 2022, Halpern led a two-person study to gauge the feasibility of using DBS to treat binge eating disorder. Subjects reported improved self-control of food intake and weight loss over the study’s six-month observation period.
“It’s like faith healer, cast aside your crutches and walk kind of stuff.”
One of the most promising results yet for mental-health-related DBS came early last year. A group based in Germany implanted electrodes in a dozen people with treatment-resistant alcohol use disorder, activating implants for only half of the patients at first. The researchers initially saw a profound placebo effect: Patients seemed to improve whether their implants had been activated or not. But differences between the groups emerged months into the trial, when the treated individuals — the so-called active group — began to maintain lower levels of alcohol use than the control cohort.
“Descriptively, the active group was performing better,” said Patrick Bach, a lead author on the work. But given the small size of the study, he said it was impossible to say just how much better — or to rule out the possibility that it was a statistical fluke.
From the beginning, the problem of small study cohorts and large statistical uncertainties has plagued efforts to determine whether DBS works for opioid use disorder, and how. Substance use diseases are difficult to study in preclinical animal models, which complicates attempts to assess and refine treatments before they are deployed in humans. And the cost, invasiveness, and novelty of DBS have made it difficult for clinicians to recruit clinical trial participants in the numbers necessary to reliably infer the efficacy of the treatment.
Years ago, when researchers at the University of Amsterdam launched a pilot study to test DBS as an opioid use intervention, they recruited participants from six facilities that typically treated about 27,000 patients each year. Three years into the study, they had received just 23 referrals, and only two patients had started the trial. Follow-up surveys of patients who didn’t participate suggested concerns about the surgery led them to drop out.

Alik Widge, a psychiatrist at the University of Minnesota, said that the small trials and one-off success stories serve as trail markers that chart the safety, dangers, and potential of DBS as an addiction intervention.
Visual: Courtesy of Alik Widge
When small trials do occur, it’s difficult to know how much of a patient’s improvement is due to the DBS itself, how much is attributable to counseling and other conventional therapies patients receive alongside the DBS treatments, and how much is due to the placebo effect — a patient’s belief that the procedure is working, whether or not the electrodes are actually switched on.
People who receive DBS for opioid use disorder might also do so by way off-label treatments: A clinician who is already performing a DBS procedure for an FDA-approved use, such as a movement disorder, will sometimes offer to implant an additional electrode to curb a patient’s addictive impulses.
That’s how Max Daly came to have electrodes implanted in his brain to treat not only his movement disorder, but also his opioid use.
During his hours-long surgery, doctors placed three electrodes in different parts of Max’s brain: two to address the movement disorder and another for addiction. This latter electrode, the only one placed off-label, was inserted in the same region of the brain that’s been explored in studies of DBS for depression. The team hoped the electrode would alleviate his symptoms of treatment-resistant depression as well. But “the primary reason we did it was because of the risk to his life from his ongoing opiate use disorder,” Halpern says. They also placed two batteries in his chest to power the implants.
Max describes the surgery as a success, if not quite a panacea. It helped reduce his symptoms of depression as well as his craving for opioids, which he says he has not used recreationally since his operation. But it has proven less helpful for his cravings for methamphetamine and cocaine, he said. “I did have a relapse while living in California and where I did end up using methamphetamine. It was shortly after the surgery,” he said. He believes the treatment is most effective when he attends AA meetings and therapy sessions. “Just relying on DBS alone, like with any addiction treatment, isn’t really enough.”
The same year that Daly received his implant, neurosurgeon Ali Rezai of West Virginia University began the first NIH-funded clinical trial in the U.S. to test the effectiveness of DBS for opioid addiction. The project aims to enroll approximately 20 patients with histories of repeated opioid overdoses and track their drug consumption and cravings with urine tests and patient surveys. Last year, the group published results for the first four patients: Two completely abstained from drug use for more than a year after the surgery; one had their implant removed because he didn’t comply with the study’s protocols; the fourth person returned to drug use, but only sporadically, using fentanyl, methamphetamine, and cannabis on several occasions during the one-year follow up period, often at times when they were unemployed or unhoused. That participant reported that the procedure didn’t diminish their cravings for opioids.
The wide range of patient outcomes in early studies of DBS for opioid addiction underscores a sobering reality for the field’s researchers: Promising results for one patient are not necessarily a harbinger of success for others.
“We need to think seriously about what one resounding case study that seems to show success can really show us,” said UCLA’s Carr, whose work in anthropology focuses on laboratories studying DBS. She says she is troubled by narratives — propagated both in the media and by some researchers in the field — that seem to extrapolate from a single person’s experience and overstate DBS’s prospects to solve public health problems.
The wide range of patient outcomes underscores a sobering reality for the field’s researchers: Promising results for one patient are not necessarily a harbinger of success for others.
“There’s a sort of looping effect with patients consuming popular media like magazines and newscasts and so on that have this sort of evangelical celebratory idea that this brain implant, with the push of a button, can fix your problem. Of course that’s appealing,” Carr said. “The question is, is the implant actually that?”
In reality, critics say, DBS is unlikely to ever solve the public health crisis posed by opioid addiction. It’s “a treatment which is incredibly expensive, requires all sorts of highly trained people to provide it, and is likely to be delivered to a minuscule proportion of people who might — in principle — benefit from it,” said neuroethicist Wayne Denis Hall, an emeritus professor at the University of Queensland.
Hall and others who work in the field of addiction research see efforts to finesse DBS into a cure for treatment-resistant forms of addiction as a misplacement of priorities, and they suggest that research dollars and public health efforts would be more impactful if spent on resources such as medications to treat overdoses and therapy for larger numbers of patients. DBS “really shouldn’t be a first line treatment,” said the University of Alabama at Birmingham’s Heinsbroek, who instead sees the procedure as a last-ditch method to be used when it is “perhaps the only way to prevent somebody from passing away by overdose.” He notes that, although the surgery is generally considered safe, placing foreign objects in the brain does carry risks of inflammation, scarring, and other side effects.


Bach, who helped lead the recent trial of DBS for alcohol use disorder, supports further study of the technique as a potential treatment for substance use disorder. He agrees that the data are not strong enough at the moment to support its use outside of clinical trials, but he says it is important to continue amassing evidence on behalf of people who have run out of other treatment options. DBS might have its place for those patients, he said, “because here we have to balance an untreated disease which is deadly and which has a high, high, high risk of mortality against a procedure that also carries risk.”
As University of Minnesota psychiatrist Widge describes it, the small trials and one-off success stories serve as trail markers that chart the safety, dangers, and potential of DBS as an addiction intervention. Each new encouraging result makes it easier to advocate for technological improvements and larger trials that could help researchers glean new clinical insights. A priority, Widge says, is unraveling why the treatment helps some people and not others, “so that we can start to understand what’s different about them” and avoid putting them through unnecessary surgery.
“There is a lot of hope around DBS for substance use disorders, and most experts would say there is biological plausibility about the general idea,” Widge wrote in a follow-up email. “I think most of us would also say that it is not yet proven, either clinically or mechanistically, and there is a lot we have to learn.”
And even if DBS as a treatment never pans out, Halpern sees each case study as progress in understanding addiction as a neurological disease rather than a moral failing. “By putting an electrode in the brain, we were able to help this patient’s opiate use disorder, which really provides substantial evidence that it is a disorder of the brain and not a disorder of will or a weakness,” he says.
Although patients with opioid use disorder have generally been hesitant to subject themselves to the rigors and unknowns of DBS treatment, some evidence suggests that could change.
In 2020 and 2021, Widge and a team of collaborators surveyed 20 people with substance abuse disorders to explore their stance on DBS as a possible treatment. Only two had heard of the treatment method beforehand, and most expressed discomfort at first, describing it as “weird” or “scary.” Substance use remains a deeply stigmatized disorder and a criminal offense in many parts of the world. Many respondents raised concerns about the privacy risks posed by a neural device that records information about their addictive impulses, noting that they would never want law enforcement officers to have access to the data. However, by the end of a 90-minute interview in which patients shared their personal experiences, learned about the science behind DBS, and discussed ethical and privacy concerns, only one of the 20 participants said they would never consider it as an option.
Newer technologies that use less invasive, less expensive, and safer DBS methods may also pave the way to broader participation in clinical trials. Halpern and his colleagues have developed implants that, rather than continually delivering electric pulses, deliver them only when they detect a compulsive urge, which mitigates possible side effects.
For people like Max Daly, life at the leading edge of an unproven therapy is hopeful, but fraught with uncertainty.
Other researchers are working on techniques that could achieve the benefits of deep brain stimulation without the need for physical implants. In 2017, neuroscientist Nir Grossman of Imperial College London and his colleagues showed they could use electrodes on the surface of the skull to create oscillating electric fields that penetrate to precise regions deep within the brain, without affecting surface structures. Last October, the team demonstrated that the technique, when targeted on the hippocampus, could improve people’s ability to pair faces and names, although the question of how and why the stimulation works remains unclear.
In the meantime, for people like Max Daly, life at the leading edge of an unproven therapy is hopeful, but fraught with uncertainty.
Following his surgery, Max graduated high school, and he now lives independently outside of Paris. Last August, he was dealt a setback: He developed a severe bacterial skin infection near the battery that powers his implant for addiction. (The battery for the electrodes that treat his movement disorder was spared.) Clinicians in Paris severed the power supply to his electrode to treat the infection. “Since then, I haven’t used, but I’ve just been extremely depressed,” Max says.
This month, Max returned to the U.S. for yet another surgery, to restart the implant, and then, hopefully, resume his life. “Max was — and will be again — completely back to normal, including enjoying arts, studying, reading,” Elena says. DBS, to her, is “not just a tool to prevent the ultimate horror of overdose and death. It’s really bringing back the life, and the enjoyment of life.”