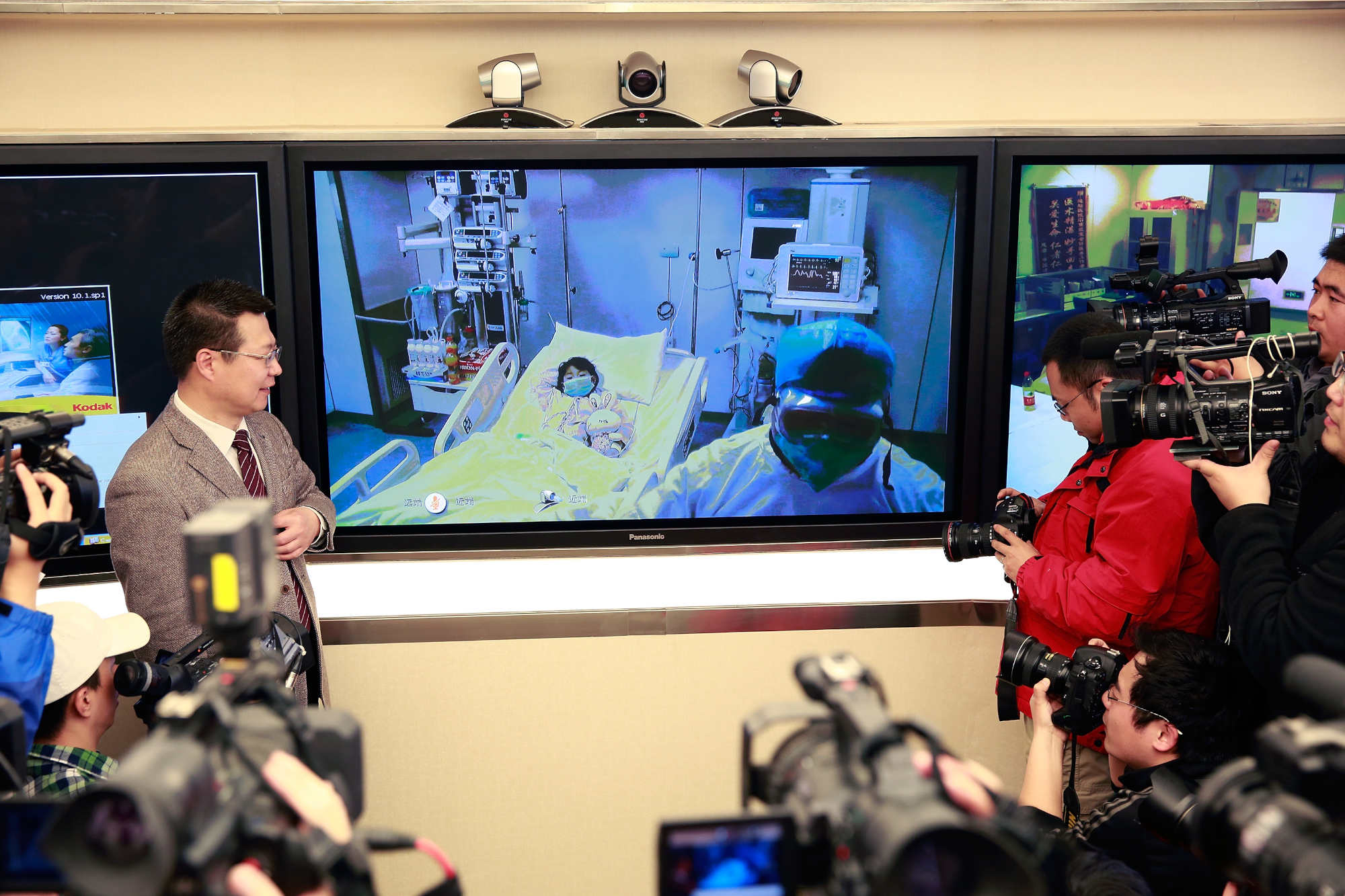
In March 2013, a 27-year-old man showed up at the Fifth People’s Hospital in Shanghai, China, with a high fever and cough. Within 48 hours, he was admitted to the intensive care unit with severe shortness of breath. But no treatment could save him, and he died four days later. Chinese health experts later confirmed that the young man, who worked as a butcher, had been infected with an influenza virus called H7N9.
His wasn’t an isolated case. Other patients were trickling into Chinese hospitals with the same infection. Research later showed most of the infected individuals had a history of recent contact with poultry. But there were documented cases of patients infecting immediate family members who hadn’t recently been exposed to poultry, suggesting the virus might have been evolving a capacity to transmit among people.
Human cases of H7N9 flu appeared periodically in China and other countries over the next several years. Between early 2013 and late 2017, there were nearly 1,600 lab-confirmed cases and roughly 600 deaths. The infections sputtered out after Chinese officials launched a national campaign to vaccinate chickens against the virus in 2017. But on the other side of the world, at Pennsylvania State University, a virologist named Troy Sutton still had nagging concerns. Sutton was investigating how influenza evolves to become highly contagious, and he often worked with ferrets, which respond to flu viruses in much the same way as people. H7N9 should have struggled to transmit in ferrets, but Sutton’s research found that the virus could sometimes infect animals housed in adjacent cages, picking up new mutations along the way.
Experts had been warning that H7N9 could have high pandemic potential if human-to-human transmission were sustained. Had Sutton uncovered genetic clues into how H7N9 might evolve to become a global threat?
In 2018, Sutton proposed a new study to answer that question: He would insert the newly discovered mutations into H7N9 and then assess the virus’s capacity to infect ferrets in the lab. In short, Sutton was intending to give the virus a “gain of function,” or GOF, that might improve its transmissibility. If the engineered virus became more transmissible, then “we would know that those specific mutations promoted transmission,” he wrote in an email. “If these mutations were then identified in other circulating H7N9 viruses, this would indicate [increased] pandemic risk.”
GOF experiments involve studying how genetic changes alter what a microbe can do. If the changes allow for new capabilities, then the microbe has gained functionality. Conversely, genetic manipulations can also produce losses of function, such as lab-adapted strains that are unable to survive in the outside world. Used routinely in biomedical research, GOF methods can reveal how pathogens evolve resistance to drugs or vaccines. Scientists use the methods to develop treatments for infectious diseases, to develop vaccines, and to create “oncolytic” viruses that are programmed to attack cancer cells.
Some experts are skeptical that any genetic changes observed from GOF experiments with potential pandemic pathogens would be useful for disease surveillance.
These run-of-the-mill GOF applications “do not have a risk of creating a pandemic or enhancing pandemic potential,” Angela Rasmussen, a virologist at the University of Saskatchewan, wrote in an email.
But that’s not necessarily true of studies that alter dangerous pathogens to assess how they might become more virulent or transmissible. Public controversies have erupted over this sort of research, but in fact few such studies have been performed so far. The research might reveal mutations that turn pathogens into bigger human health problems. Armed with that knowledge, scientists could look for those genetic changes during disease surveillance, with an aim to identify and prepare for looming outbreaks.
It can be hard to find definitive evidence that knowledge of those mutations will, in fact, help predict or prevent the next pandemic. Indeed, some experts, are skeptical that any genetic changes observed from GOF experiments with potential pandemic pathogens would be useful for disease surveillance. And in the worst-case scenario, experimenting with viral genomes might create an even more dangerous virus that escapes into the world. Those concerns came to the fore during the Covid-19 pandemic, when U.S. government officials were accused of supporting GOF studies with coronaviruses at the Wuhan Institute of Virology. The pandemic started in the city of Wuhan, and whether it resulted from a lab-escaped virus or a viral “spillover” from wild animals to people has yet to be conclusively determined.

Marc Lipsitch, an epidemiologist at the Harvard T.H. Chan School of Public Health, said that regardless of whether someone blames Covid-19 on a lab leak or a natural event, this calamity showed current generations that a global pandemic is “not a storybook or science fiction concern.” Lipsitch acknowledges that GOF is undeniably a valuable technique for microbiological research. But he argues that GOF experiments with highly pathogenic strains are justifiable only under narrow circumstances, such as trying to understand how a widespread virus, like SARS-CoV-2, that is already “infecting millions of people daily,” escapes the immune system, he said.
During a recent global survey, infectious disease specialists ranked influenza, SARS-CoV-1, SARS-CoV-2, and Ebola viruses as today’s top pandemic threats, given their evolutionary potential, transmissibility, and histories of causing large-scale outbreaks. GOF research might help scientists anticipate which genetic changes could drive dangerous pathogens to become more prevalent — or it might not. Is that elusive payoff worth the risk?
As a descriptive term, GOF has never been all that popular with scientists. “It’s a terrible, terrible term that was really created, you know, just to pick something,” said Gigi Gronvall, an immunologist at the Johns Hopkins Bloomberg School of Public Health. Although the term appears in some earlier research, it made its way into the wider public consciousness when it was used to describe two controversial experiments that increased the transmissibility of avian influenza. Other terms, including “gain-of-function research of concern” and “enhanced potential pandemic pathogen research” are also used frequently and interchangeably.
A gain of function can be induced in several ways. One option is to simply grow a virus in iterations. Called serial passage, this method involves successively transferring a virus from one group of cell cultures or lab animals to another. The virus evolves genetically with each passage, acquiring mutations that help it adapt to a new host. Scientists can then determine which mutations allow for functions of interest, such as transmission among lab animals through the air or resistance to the host’s immune system. Serial passage can be viewed as a means of discovering mutations and other genetic changes that provide a virus with new capabilities. Alternatively, scientists might suspect at the outset that certain mutations boost pathogenicity. They can test their hypotheses with GOF experiments that involve engineering mutations directly into the viral genome. Scientists may also co-infect animals with different viral strains that might combine, or reassort, into an entirely new virus.
One infamous example of GOF research with potential pandemic pathogens — one that sparked long-standing efforts to regulate the experiments — dates back to when a pair of laboratories, one in the U.S. and one in Europe, conducted studies with an avian influenza virus that was decimating poultry and infecting wild bird populations around the world. The type of influenza viruses that are known to cause human pandemics are categorized by two protein spikes that help the microbe invade other cells: hemagglutinin (H, also called HA) and neuraminidase (N, also called NA). There are many H and N subtypes, and each one is numbered. The research in this case, published in 2012, was focused on H5N1 influenza. Human H5N1 cases were rare — only 600 or so people globally had been infected with the virus from 1997 to early 2013 — but they had an estimated fatality rate of more than 50 percent, though experts say that may be exaggerated. Virologists were heavily divided as to whether H5N1 could evolve to infect mammals without losing the fitness it needs to survive. But scientists and government officials worried that if H5N1 did evolve to spread efficiently in people, it would pose a global threat.
To investigate, a virologist named Yoshihiro Kawaoka at the University of Wisconsin–Madison performed a controversial experiment. With funding from the NIH, Kawaoka and his team created a genetic hybrid that combined a mutated hemagglutinin from H5N1 with genes from a strain of H1N1 influenza that killed hundreds of thousands of people during a roughly year-long pandemic that started in 2009. After acquiring just a few additional mutations, that lab-created virus could spread through the air, sickening ferrets in adjacent cages.
Meanwhile, at Erasmus Medical Center, the virologist Ron Fouchier was investigating H5N1 in a different way. Also with NIH support, Fouchier used serial passage to transfer a genetically-modified Indonesian strain of H5N1 repeatedly in ferrets. And like Kawaoka, he produced a virus that could infect animals by an airborne route. The evolutionary change had required as few as five additional mutations, with no need for reassortment with any other flu virus. Taken together, the two studies provided alarming evidence that H5N1 was potentially only a few mutations away from being able to spread easily among people. (Through media representatives, both Kawaoka and Fouchier declined requests for an interview with Undark.)
In a 2011 article in Science magazine published in advance of his paper, Fouchier acknowledged that his team had created “probably one of the most dangerous viruses you can make.” NIH officials had apparently decided to fund the studies after determining they were well designed. “This wasn’t a thing where, you know, a couple of scientists went off on a wild hair,” said Ralph Baric, a virologist at the University of North Carolina, Chapel Hill. “This was a heavily discussed topic at the highest levels of health agencies across the globe.” Anthony Fauci, then the director of the National Institute of Allergy and Infectious Diseases, was quoted on the NIH website saying that benefit to public health from fully publishing the papers “far outweighs the potential risks.”
For all of Undark’s coverage of the global Covid-19 pandemic, please visit our extensive coronavirus archive.
The studies generated an uproar, and some experts opposed their publication, fearing terrorists or others with bad intentions might use the results to create a bioweapon. Rebecca Moritz was a manager for the University of Wisconsin–Madison’s select agent program — her job was to ensure that microbiology studies complied with federal regulations for highly infectious materials. She noted that Kawaoka’s team “did everything and more” to adhere with the CDC’s best practices for safe lab research, working under the appropriate biosafety level, or BSL.
The bottom rung, BSL-1, is typically reserved for work with low-risk microbes and doesn’t require special containment equipment. Under BSL-2 protocols, technicians work behind self-closing, lockable doors and wear personal protection equipment such as lab coats, eyewear, and sometimes face shields. Kawaoka and his team worked under BSL-3. According to Moritz, they wore scrubs, two pairs of gloves, dedicated shoes, shoe covers, and respirators equipped with air-purifying filters. BSL-4 protocols, which are reserved for the most dangerous pathogens, such as Ebola, may require technicians to wear full-body protective suits connected to their own air supply.
These protocols aren’t fool-proof. And as the old saying goes, accidents happen. Researchers can — and have, in the past — become infected while working with viruses in the lab, especially if they don’t use protective equipment correctly. Pathogens have also escaped labs in other ways, such as through leakage of infectious waste water and accidental shipping of infectious samples. Earlier this year, the Bulletin of the Atomic Scientists published a report citing more than 2,300 lab-acquired infections across all biosafety levels between 1979 and 2015. More than 300 such incidents — including eight deaths — occurred during first two decades of this century, suggesting that “modern practices and containment may reduce risks, but cannot eliminate them completely,” the report’s authors wrote.

Could the Kawaoka and Fouchier viruses have caused a disease outbreak if released accidentally? “That’s the million-dollar question,” Sutton responded. Some viruses that transmit in ferrets will not transmit in people, Sutton pointed out, so while genomic changes that boost airborne transmission “certainly indicate increased pandemic risk,” scientists can’t be sure of whether accidental release of the viruses in these instances would have caused an outbreak, let alone a pandemic.
Rasmussen emphasized that the airborne virus generated by serial passage in Fouchier’s lab was less virulent than the virus naturally is in the wild. The infected animals looked sick — they were lethargic, had ruffled fur, and poor appetites, but none of those infected via airborne transmission died. “It’s something to consider,” she said, adding that scientists aren’t necessarily making “some sort of, you know, undefeatable supervirus.” Michael Imperiale, a virologist at the University of Michigan, agreed that even if serial passage selects for new traits such as airborne transmissibility, it might also select for different traits that make the virus less virulent. However, “you can’t assume that there’s always going to be a tradeoff” or that those traits are genetically linked, he added. For instance, SARS-CoV-2 naturally evolved into more transmissible variants as it moved globally from person to person, but the pathogen’s virulence “really didn’t change much,” Imperiale said. Recent evidence suggests that a recently-evolved SARS-CoV-2 variant is less virulent than those that came before.
Could the Kawaoka and Fouchier viruses have caused a disease outbreak if released accidentally? “That’s the million-dollar question,” Sutton responded.
Disputes over the public health value of the H5N1 research — and over GOF studies with potential pandemic pathogens more broadly — continue today. Baric said the research identified mutations and other biological features that allow H5N1 to transmit more efficiently between adjacent ferrets. An email to Undark that Renate Myles, director of the NIH Office of Communications and Public Liaison, requested to be attributed to a spokesperson, noted that because of prior ePPP projects on HHN1 avian flu, scientists know which mutations “represent a greater threat to humans.” Lipsitch, however, questions if the mutations identified during GOF experiments in the lab are also likely to happen in nature. Viruses in the wild, he wrote in 2018, are subject to other pressures that can force genetic changes along a different path. “There’s an argument against doing the work because it’s not generalizable in a meaningful way,” he told Undark. And the H5N1 research, he asserted, didn’t provide any new information that could have been used for developing countermeasures against H5N1 influenza in humans.
Sutton’s view is that the specific mutations identified in GOF research matter less than how those mutations change the biology of a virus. Kawaoka and Fouchier identified different mutations, but their effects on H5N1 transmission were comparable, and importantly, all relevant mutations were located in the same place: the hemagglutinin protein. Now, when a new H5N1 virus emerges, researchers look for mutations in that protein, Sutton wrote in an email. And viruses that have those mutations, he noted, “are prioritized for vaccine development and risk assessment.” Still, the downside of the research, Baric said, is that it produced a published template for how to increase flu transmission between ferrets — one that some scientists worried could have wound up in the wrong hands.

The two publications left policymakers on edge, fueling what some now describe as a negative knee-jerk reaction against almost all respiratory pathogens that have been genetically manipulated. After a series of unrelated biosafety incidents, the U.S. government announced a pause on new funding for GOF research with certain potential pandemic pathogens in 2014, which held up projects at numerous labs around the United States. The pause was eventually lifted in 2017, but then the Covid-19 pandemic reignited tensions. Sen. Roger Marshall, a Kansas Republican, introduced legislation to restrict GOF studies with potential pandemic pathogens, and a number of states took similar steps.
GOF studies are still ongoing around the world, with U.S.-affiliated investigators contributing roughly half the published studies, according to a 2023 review. However, studies that might generate enhanced potential pandemic pathogens occur rarely. “There have been very, very few examples,” Imperiale said. No such studies are currently being funded by the NIH, according to the email sent to Undark by the NIH’s Renate Myles. Much of the ongoing GOF research is devoted to biomedical applications — not just with viruses but also bacteria and fungi. Scientists might tinker with microbial genomes when testing or making new vaccines, for instance, or exploring how different pathogens become drug resistant.
In a 2023 commentary published in the Journal of Virology, more than 100 co-authors listed what they claimed are examples of useful GOF experiments. The list includes applications such as cancer therapeutics, developing microbes for hazardous waste cleanup, and engineering more resilient crops. But it also lists more controversial research with potential pandemic pathogens — including evidence that lab-altered strains of H5N1 transmit among mammals and a 2015 study from Baric’s lab showing that SARS-like coronaviruses in bats pose a human threat. The authors acknowledged that changing a virus to add new functions could yield a dangerous pathogen. But these sorts of experiments are rare and regulated, they emphasized, and meanwhile, congressional efforts to further restrict GOF “add fuel to an anti-science, fear-based movement.”

Ralph Baric, a virologist at the University of North Carolina, Chapel Hill, has studied SARS-like coronaviruses that pose a threat to humans. He worries the restrictions on the field could shift virology research to countries where regulations aren’t as stringent.
Visual: Jon Gardiner/UNC-Chapel Hill
Many of the same authors published another Journal of Virology commentary in 2024, positing that recommendations to modify and expand oversight over virology are having a chilling effect on the field. Baric worries the restrictions could shift virology research to countries where regulations aren’t as stringent, potentially depriving the U.S. biotechnology sector of innovations from academia. “I have colleagues who study coronaviruses or flu who have had to halt their research for up to six months while the research undergoes review for risk,” said Felicia Goodrum, a virologist at the University of Arizona College of Medicine and a co-author on both commentaries. “And of course, nobody really wants to do that when you have graduate students who have got to get through their Ph.D., and their research has to move forward.”
When asked about the commentaries, Lipsitch responded that those who advocate for relaxing or minimizing GOF regulations are very vocal and keep publishing very similar articles over and over again. “I think they’re influential within ASM,” he added. The articles “typically say, first of all, this is a problem for us virologists to settle — public stay out, which I think is wildly inappropriate. They even say of other scientists that they are unqualified — as they have said of me and some others.”
While scientists and public health officials weigh the value of studying GOF in the lab, several viruses now in circulation are showing worrisome signs of becoming more contagious. Sutton points to alarming trends with H5N1, which was detected in dairy cattle for the first time earlier this year. The virus has so far infected more than 60 people in the United States, most of them dairy and poultry farm workers, although three infected individuals in North America had no known immediate exposure to animals. The World Health Organization documented 939 human H5N1 cases in 24 countries between Jan. 1 2003 and Nov. 1 2024, and nearly half of them were fatal. Every new human infection gives H5N1 a chance to develop mutations that favor person-to-person transmission.
Moreover, Sutton said, new infections boost the likelihood that H5N1 might also reassort with a human influenza virus to generate a more transmissible strain. Meanwhile, H5N1 viruses have spilled into other mammals, including foxes, skunks, raccoons, minks, and seals, which “suggests the virus has evolved some degree of transmissibility in these species,” Sutton said in an email. GOF research, he said, could focus on transmission and how the virus evolves resistance to vaccines and antiviral drugs, allowing researchers to “prepare fully for the possibility of rapid spread through humans” and the potential for a pandemic. Imperiale takes a more cautious stance, arguing that health authorities might simply begin stockpiling H5N1 vaccines as a hedge against wider spread.

Similarly, the mpox virus (previously called monkeypox) “is becoming increasingly educated with humans,” said Grant McFadden, a virologist and retired professor at Arizona State University. Mpox is rarely fatal, but it produces painful rashes and sores accompanied by flu-like symptoms; infections generally last up to a month. In August 2024, the WHO declared an mpox outbreak in the Democratic Republic of the Congo and other African countries to be a public health emergency of international concern. Mpox spreads mostly by physical contact, but “my biggest worry is that as the virus becomes acclimatized and knowledgeable about humans, it will become more infectious,” McFadden said. “I would not want to see that virus become airborne.”
McFadden suggested that if scientists wanted to study how mpox evolves greater virulence and transmissibility by passing the virus between monkeys, that research should be done under high levels of biosecurity, perhaps even BSL-4. But in an era of heightened scrutiny over GOF, that sort of research faces challenging obstacles. Bernard Moss, a virologist who heads NIAID’s Genetic Engineering Section, has been studying mpox for years. As part of his research, Moss has swapped genes from different mpox variants, which are called clades, to test how lethal they are. Science magazine reported in 2022 that he was planning to insert genes from a clade 1 virus into a more virulent clade 2 virus to see if that would make it less deadly. That research was never carried out, according to information the NIH shared with Republican members of the House Energy and Commerce Committee, detailed in a June 2024 report. In a statement on the report’s release, several Republicans claimed that in approving the experiment, NIAID, HHS, and the NIH had demonstrated “a disturbing lack of judgment.” Moss didn’t respond to an email from Undark. NIAID declined to make anyone available for an interview, but the email sent to Undark by Myles at the NIH noted: “To be clear, NIH has never conducted experiments that would make monkeypox virus (MXPV) more dangerous.”
GOF studies are still ongoing around the world, with U.S.-affiliated investigators contributing roughly half the published studies, according to a 2023 review.
Matthew Aliota, a virologist at the University of Minnesota, said researchers have a choice. They can wait for more virulent or transmissible viruses to become predominant circulating strains, or try to identify the enabling mutations in advance and then look for them in surveillance networks. In his own research, Aliota and his collaborators have shown that Zika virus, a cause of human birth defects and symptoms such as rash and fever, becomes more virulent when passaged among mice in the lab. Zika spread throughout the Americas between 2015 and 2016, infecting hundreds of thousands of people, though those numbers may be underreported. The virus is transmitted by mosquitoes, but it can also be passed on sexually. Aliota found that mouse-adapted strains, which he used to model sexual transmission, became more dangerous: When Aliota’s team exposed mice to these viruses in the lab, the animals all died. Zika viruses carrying mutations that drive this heightened pathogenicity “already exist out in nature,” he said. In an email, Aliota said those are the variants that scientists may want “to monitor more closely.”

Matthew Aliota, a virologist at the University of Minnesota, has shown that Zika virus, spread throughout the Americas between 2015 and 2016, becomes more virulent when passaged among mice in the lab.
Visual: Courtesy of Matthew Aliota
Still, what researchers need to ask before initiating such a study is whether it addresses an important medical or public health issue, Imperiale said, and whether there are equivalent alternative approaches. “Do we really need to do this experiment? And I think the fact that these types of experiments have been done so infrequently says most of the time, ‘no,’” he said. Furthermore, obtaining insights for surveillance must also be balanced against the risk of lab accidents. In an email, Goodrum insisted that “biosafety and biosecurity has always been and remains at the forefront of any virology lab — particularly in the US.”
But some BSL protocols in different parts of the world are “hugely different,” Baric pointed out. Baric collaborated with Shi Zhengli from the Wuhan Institute of Virology, among others, on research showing that a coronavirus hybrid could infect human airway cells. The virus combined genes from two sources: a bat virus called SHC014-CoV and another coronavirus called SARS-CoV-1, which infected more than 8,000 people after emerging from China in the early 2000s. SARS-CoV-1 had an approximately 10 percent human fatality rate, and Baric and his co-authors’ findings, published in 2015, suggested that other coronaviruses in bats might also pose similar threats. Baric’s team conducted the study under BSL-3 conditions in Chapel Hill, North Carolina. But then Shi’s lab continued culturing bat coronaviruses under BSL-2 conditions, which are less stringent. That research has since fueled allegations that SARS-CoV-2 spawned the Covid-19 pandemic after escaping from WIV.
Many virologists insist that the strongest evidence supports a natural spillover of the virus from animals into people. In an email to Undark, Gronvall connected the lab-leak theory to “manufactured controversy” over U.S.-supported research at WIV. But during a Congressional interview earlier this year, Baric also said it was irresponsible for Shi to work with coronaviruses under BSL-2, adding that this was “one of the main reasons why I felt that the potential laboratory escape hypothesis shouldn’t be, in essence, put under the rug.”
Biocontainment labs are rising in number all over the world. And Donald Burke, a retired epidemiologist who formerly directed a BSL-3 lab at the University of Pittsburgh, believes accidental releases are more likely during times of anxiety over a looming threat. It’s during the crunch of an emergency, he said, that people end up engaging in riskier activities than they otherwise would. Furthermore, there’s a good chance that such a release will happen “outside the United States, not inside the United States where we pay more attention to these things,” Burke said.
“There are now two dozen countries in the world that have BSL-4 laboratories who ostensibly can work on dangerous pathogens,” he added. “Almost all of them want to do the right thing, but they’re often resource-constrained.”
“Biosafety and biosecurity has always been and remains at the forefront of any virology lab — particularly in the US.”
Meanwhile, back at Penn State University, Sutton still can’t say that the mutations he proposed to study would have enhanced the transmissibility of the H7N9 virus. After what he describes as a very tiered and very thorough risk mitigation review — one that took three years — Sutton’s original proposal was denied. Spooked by Covid-19 and controversies over the possible lab-leak, NIH officials would only allow him to work with a weakened strain of the virus. The unpublished results, obtained from experiments in petri dishes instead from live animals, identified mutations that confer changes, Sutton said. “But at the end of the day, we still don’t know — I still don’t know — if these changes we identified will make the virus transmissible,” he said. “That’s the piece that’s missing.”
The mounting restrictions have led Sutton to change how he thinks about science. “You have to think, well, what can I do?” he asked. “What experiments can I do to get at this? Because you have to change your approach. You have to come at it from a different angle. And for me, it has changed my approach to some of our work, because I’m not conducting, planning, or prepared to do gain of function research at this time.”











Just how long would it take for these viral pathogens to “advance” on their own? It might, if ever, possibly take decades, maybe centuries absent the scientist playing these rounds of “GOF.” It seems that these researchers think that the human genome remains static through natural evolutions. Apparently they seek to be the heroes of the pandemic they lust for, to say nothing of the lucrative nature of these unnatural discoveries. A (monkey?) pox on them .
I think the defensive application of this research is total BS. They make pathogens that kill people. That is all.