An Unorthodox Allergy Clinic Seeks to Disrupt Medicine
Carrie Martin had a two-part system to protect her food-allergic daughter from accidental exposures to peanuts or sesame. Martin scoured ingredient lists at the grocery store. Her husband re-checked them at home before cooking meals. “We thought our system was so foolproof,” said Martin, a teacher in Ormond Beach, Florida.
But once, in 2012, Martin unwittingly grabbed the wrong pizza crust at the grocery store. After her daughter coughed, threw up, turned red, and got treated in the emergency room, they pulled the wrapper out of the trash and realized why — the pizza had contained sesame. Years later, in 2018, the family experienced another scare. Some restaurant-baked bread sent their daughter into anaphylaxis, a life-threatening allergic reaction that required two epinephrine injections and a trip to the hospital.
“It’s like you’re back to ground zero again,” Martin said. A single reaction can plunge even the most vigilant food allergy families back into what-ifs and panic.
Six months after the restaurant emergency, Martin found herself perusing the website of a promising — yet decidedly unorthodox — allergy clinic located in Long Beach, California. Martin had never been one to seriously explore treatment options, but the Southern California Food Allergy Institute advertised something different. Not only did this institute offer hope beyond a life of EpiPens and strict avoidance — it claimed that patients who finish treatment can freely eat the foods they were allergic to.
Until last year, the Food and Drug Administration had not approved any treatments for food allergies, which afflict an estimated 32 million people in the United States. By the late 2000s, a handful of pioneering allergists were starting to use a procedure called oral immunotherapy (OIT), which tames a person’s allergies through daily ingestion of small amounts of store-bought foods. But the procedure is not a cure. For the most part, it can train the body to tolerate, say, two peanuts or a half glass of milk — just enough to keep accidental exposures from escalating into the serious reactions Martin’s daughter experienced.
Over the past decade, the OIT cottage industry has grown to about 200 practitioners, according to one allergist’s estimate, and their private clinics have treated more than 15,000 patients. Some allergists have started using standardized peanut-powder capsules that work on the same low-dose exposure principle after the FDA approved their use in January 2020. Still, most U.S. board-certified allergists do not offer food immunotherapy. The treatment remains controversial because it’s time-consuming, can trigger allergic reactions, and doesn’t work for everyone. In some studies, OIT treatment was unsuccessful in 15 to 30 percent of patients.
SoCal Food Allergy, as the institute is popularly known, grew out of a one-man operation that started in the basement of a nearby hospital in 2004. The institute offers individualized treatment using a method that some allergists say resembles OIT but which its founder argues is fundamentally different. Unlike OIT, which exposes patients to incrementally increasing amounts of their allergens alone, SoCal’s program leans on mathematics and machine learning to determine customized dosing schemes using a range of foods. The goal is to build patients’ tolerance to related foods and then introduce the allergen once their immune systems are less reactive.
According to figures provided by the institute, at least 11,000 such patients have been treated or are being treated with SoCal’s Tolerance Induction Program, and more than 5,000 have reached remission — meaning they can “eat whatever they want, whenever they want, in unlimited quantities, without fear of reaction.” SoCal Food Allergy reports a 99 percent success rate for its program, with a side effect rate considerably lower than those reported for private-practice oral immunotherapy.
Martin put her daughter on the SoCal waitlist in January 2019. Roughly 1,350 families were ahead of her, and Martin was told the wait would be about 13 months. “I was totally fine with that,” she said.
As Martin’s daughter moved up the waitlist, though, she began to panic about logistics and cost. The thought of committing her child to a treatment that required cross-country trips every few months weighed on Martin. SoCal Food Allergy has an annual program charge of $4,500. According to parents of program participants, this fee was originally considered a donation, briefly became a mandatory “tax-deductible contribution,” and now is a non-tax-deductible fee. And by 2019, the institute had switched to in-house lab testing that was not yet covered by all insurers, forcing families to pay thousands of dollars out of pocket for a set of required tests that used to be done by companies such as Quest Diagnostics and were usually at least partially covered by insurance.
Martin also had doubts about the institute’s self-reported results. “It seemed,” she said, “too good to be true.”

Allergists have expressed similar skepticism about SoCal Food Allergy’s reported results. “Come on, there’s no way,” said Hugh Windom, whose Sarasota, Florida clinic has treated about 500 patients with oral immunotherapy since 2012. Philippe Bégin, an allergist and researcher at the University of Montreal in Canada, called the 99 percent success “just ridiculous.” And while Edwin Kim, an allergist and immunologist at the University of North Carolina, said he wanted to “give them the benefit of the doubt,” he added: “I’d be lying if I didn’t say I’m skeptical.”
In interviews with roughly a dozen allergists, many took issue with the length and expense of SoCal Food Allergy’s program and its lack of peer review, which makes it hard to understand how the proprietary method works and how effective it really is.
SoCal Food Allergy’s founder, Inderpal Randhawa, acknowledges the skepticism. Describing his institute’s approach, he said, “It’s very unusual. I get it, most people don’t work like I do. They don’t have my mindset.” But as a multi-specialty physician-researcher, he sees a gap in the field that he believes he can address. Randhawa argues that his method eludes peer-reviewed publication because its machine-learning algorithms are groundbreaking and ahead of their time.
“I’m on a mission, point blank,” he said. “Right now, the system is antiquated,” he said, speaking about the field of medicine broadly.
Only in the last few decades have researchers started to treat food allergies with the same desensitization approach used for more than a century to treat environmental allergies such as pollen and dust mites. There are various reasons. While watery eyes and sniffles triggered by environmental exposures are considered an annoyance, allergic reactions to food are potentially deadly. And though food allergy deaths are rare, when such incidents occur during a treatment trial, they can stall research for years, said allergist Sakina Bajowala of Kaneland Allergy and Asthma Center in Illinois. In 1991, an error caused a study participant to suffer a fatal allergic reaction to peanut, sending shockwaves through a community whose practitioners are by nature very cautious.
Only about one third of allergists surveyed in 2018 and 2019 said they administer, in any given month, more than five food challenges — a process that involves giving patients increasing amounts of a food and watching for potentially life-threatening allergic reactions. These procedures are the gold standard for diagnosing allergies, yet clinicians use them sparingly. And many shy away from offering oral immunotherapy.
This reluctance is also tied to the current regulatory framework. In order for a medication to receive FDA approval, its manufacturer must first prove that the drug is both safe and effective. This is a long, expensive process that includes testing the medication on human patients. Yet most OIT involves simple grocery store fare — milk, eggs, wheat — so it lacks the funding and standardization required for testing. And without FDA approval, insurance companies are less likely to cover the procedure.
This is the world Randhawa entered in 2004, when he started seeing food allergy patients in the basement of Miller Children’s and Women’s Hospital in Long Beach. Randhawa says he took careful histories and analyzed immune cells and proteins in blood samples he collected from patients to answer basic questions: What doses of which proteins cause allergic reactions? What doses can change levels of reaction-triggering immune proteins in a patient’s blood?
Undaunted by applied math and mounds of data, Randhawa says he analyzed “weighted effects of different proteins” and raised questions that hadn’t gotten much traction among allergists — for example, if you eat a walnut and happen to be allergic to peanuts, what does the walnut do to you? How might a person’s allergic responses be shaped by proteins with similar structures found in other foods or drugs or even in the environment?
The program Randhawa developed was a natural outgrowth of his curiosity and multidisciplinary training. After graduating magna cum laude with a bachelor’s degree in biochemistry at the University of Southern California, Randhawa enrolled at Northwestern University’s medical school in 1997. He trained in pediatrics and internal medicine, and then from 2005 to 2009, completed two fellowships, the first in allergy and clinical immunology, and the second in pulmonology, where he treated lung diseases.

Inderpal Randhawa, founder of the Southern California Food Allergy Institute.
During his first fellowship, Randhawa helped with a large study testing whether Xolair, an asthma drug manufactured by the biotechnology company Genentech, could prevent reactions in peanut-allergic children. As had happened in the 1991 peanut allergy study incident, a fatality at one of the Xolair sites “basically shut down the entire nationwide trial,” he said. Doctors and scientists started thinking that trying to treat food allergies was just too risky, said Randhawa, who lists faculty appointments at the UCLA David Geffen School of Medicine and UC Irvine on his CV, among numerous other positions. Randhawa also serves as medical director of the Children’s Pulmonary Institute at Miller Children’s & Women’s and program director for its cystic fibrosis center. “People were saying, ‘well, this is too risky, just don’t do it. Don’t touch this field.’”
But after seeing children die of food anaphylaxis during his residency training, Randhawa says he couldn’t turn away. In the intensive care unit one night, two kids were recovering from anaphylactic reactions to similar amounts of peanut, he recalls. One was on oxygen and seemed to be stabilizing. The other lay brain dead, hooked to a ventilator. Yet when Randhawa looked up their charts, he was stunned to discover that the child on oxygen had markedly worse allergic numbers than the one who was brain dead.
Randhawa said the other physicians shrugged away the finding, but he was flabbergasted. It “just didn’t make sense to me,” he said. The stark disconnect between metrics and outcomes resonated with an earlier personal tragedy: the sudden death of his father in 2000 from a massive heart attack, despite four negative stress tests the previous year and no significant risk factors. “All of my time in medicine and science since has forced me to question and develop ideas well beyond my peers,” Randhawa wrote in an email. “This is why I am motivated and able to build faster and wider across multiple fields.”
As he began to question conventional protocols for diagnosing and treating life-threatening food allergies, Randhawa says he studied the physiology of anaphylaxis. He looked at immune cell populations and pathways to understand how different organs and their cells react to food. He combed through agricultural chemistry journals. He pored over plant protein databases, searching for patterns and structures, known as epitopes, in foods.
Drawn to surgery and the high-intensity ICU during his allergy and immunology training, Randhawa studied food allergies with a transplant mindset. To determine if someone can receive an organ transplant, doctors need to make sure the recipient’s immune system won’t reject the donor organ. Transplant surgeons compare signature molecules on the surface of cells of the donor and the recipient to be sure they match well enough.
In a similar vein, food allergies occur when specific molecules in foods prove to be a poor match for a person’s immune system. Randhawa believes that over time, the immune system can be conditioned to become a better match — to tolerate its allergens — through a progression of increasing exposures to related proteins in less-triggering foods. These principles, he says, form the basis of his treatment program.
“I will put whatever amount of energy and time and money,” he said, into seeing this through.
Randhawa’s program now occupies a four-story, 30,000-square-foot institute with clinic rooms, research space, and a full-fledged testing facility — and 100,000 square feet in five additional facilities within a six-mile radius. In the last few years, SoCal Food Allergy has expanded from about 40 to more than 200 employees. Operating in conjunction with the Translational Pulmonary and Immunology Research Center — a nonprofit center founded to develop individualized treatments for rare diseases including bronchiectasis and cystic fibrosis — the food allergy institute sees something like 100 to 150 new patients a month.
Inside, researchers in white coats transfer patient samples into test tubes, technicians prepare vials of peanut butter for food challenges, and patients are greeted by personalized messages adorning the crinkly paper draped over exam beds.
What’s less apparent to casual observers is that the staff treat patients with a proprietary protocol that remains shrouded in mystery. “I went on their website and still don’t understand what they do!” one physician wrote on a recent email thread among allergists offering food allergy immunotherapy. “It’s a bit of a black box,” another allergist replied.
To reach remission, patients must adhere to a customized plan that lasts months, often years. Treatment schemes are uniquely built for each patient using mathematical algorithms created by Randhawa and his team of data scientists and statisticians. The algorithms analyze hundreds of molecular markers — including antibodies that recognize specific parts of food and environmental allergens — in each patient’s blood sample.



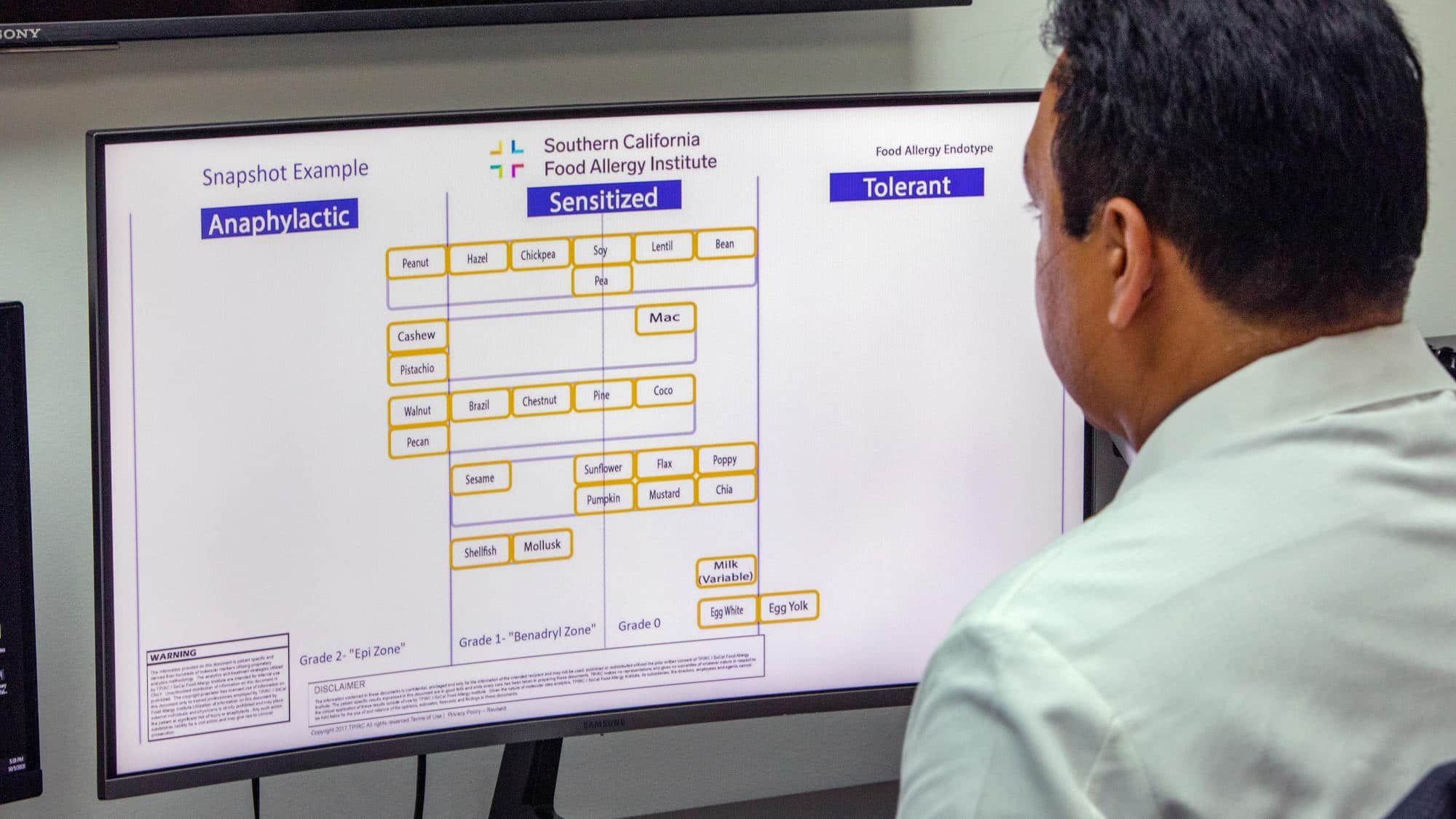
The number crunching generates for each patient a “snapshot” that classifies dozens of foods as anaphylactic, sensitized, or tolerant. “Anaphylactic” foods trigger allergic reactions if eaten in tiny amounts. On the other extreme, “tolerant” foods can be consumed freely without worry. Foods in the middle “sensitized” column sit in a gray area. According to the TIP Welcome Packet, moderate amounts of these foods produce “allergic pathways and proteins” in the blood yet may or may not cause an allergic reaction.
A person’s molecular data also factors into the treatment plan, down to the order of allergens treated and the milligrams of food protein for each dose. The goal is to move each food, one by one, into the “tolerant” column — with tolerance confirmed through in-office food challenges.
For Martin’s daughter, that meant starting the program with challenges to coconut and pumpkin seeds, foods she has eaten with no problem. “I couldn’t understand wasting the time and money to challenge two things she eats on a regular basis,” said Martin. “It just didn’t sit well with me.”
This aspect of the approach also raised suspicion for Shannon Hill, a San Diego-area lawyer whose 3-year-old son is allergic to peanuts and wheat. “The program claims that treating ‘sensitized’ and related foods first makes it safer to treat the actual allergen,” she wrote in an email. “It also adds years to treatment plans.” SoCal Food Allergy offered Hill’s son a five-plus-year regimen that included dosing with several foods he already eats regularly with no problems. For the first year, she said testing, supplies, and the annual fee would have cost about $9,000.
Still, Hill opened herself to the possibility that SoCal Food Allergy’s testing could resolve lingering questions about suspected allergies to foods her son had not yet eaten. To better understand his test results and plan, Hill searched for publications supporting the theory that pre-treating with “sensitized” foods makes it safer to treat a patient’s allergens. Finding none, she emailed and phoned SoCal Food Allergy’s doctors.
Hill found their answers vague and unsatisfactory. “It seemed like, ‘here’s a protocol and you have to follow it, and there’s no deviation, even if it doesn’t really make sense,’” she said. “The cynic in me, and maybe the lawyer in me, is like, ‘I need to see evidence.’” Hill eventually withdraw her child from the program, as did Martin.
Others have balked at having to pay huge fees for tests other allergists don’t typically request. “At one point I wondered, is it for his data?” said Alvina Leung, a Kaiser physician living in Huntington Beach, California who kept her son under Randhawa’s care for six years. “I felt like I was at his mercy.” Her son cleared his milk, egg, and tree nut allergies but kept having “reactions out of the blue” while trying to complete the peanut allergy program, Leung said. Eventually, Leung said, her son was discharged from the program without having fully completed it. “It was a very rocky road.”
Some families appreciate the rigor and rationale of SoCal Food Allergy’s method, and the institute’s Facebook page includes a number of glowing reviews. Siddharth Mallick, a mechanical engineer in Houston, learned about the SoCal institute in 2015 after his son’s struggles with traditional OIT. On the first OIT attempt, his son Anurag — who is allergic to dairy, peanuts, tree nuts, and some legumes — threw up for three weeks and dropped 20 percent of his body weight. Several months later he gave it another try, but the vomiting returned, along with lip and ear swelling and gastrointestinal issues. That summer Anurag stopped OIT and attempted six months of herbal therapy in New York before having to quit that as well, due to frequent reactions.
Shortly after stopping OIT, in August 2015, Mallick posted about his son’s setbacks in food allergy Facebook groups, and another parent suggested SoCal Food Allergy. That family had an allergen list “way longer than ours” and more complications and similar GI issues, Mallick said. He and his son decided to give it a try: “What’s the worst that can happen?”
In February 2018, two and half years after joining the SoCal Food Allergy waitlist, Mallick and his son flew to Long Beach and started the program. To treat his dairy allergy, Anurag started by dosing with boiled mare milk. Its proteins are similar to those found in human breast milk — something he could tolerate. From there, he moved on to raw mare milk, sheep yogurt, goat milk, and raw camel milk. “We call it the ‘petting zoo,’” Mallick joked.
These tailored progressions are key to the program’s success and safety, Randhawa says. Each patient’s dosing sequence for a given allergen is computed from measurements of how their immune system binds to various classes of proteins in that food. Pre-treating with related proteins from foods they already tolerate helps condition the body for dosing the actual allergen. “They’re already 50 percent, 75 percent less anaphylactic to it before we even start,” Randhawa explained in a live Q&A event that was posted online.


Tricia Morphew, a biostatistician who has worked with Randhawa on research projects spanning more than a decade, wrote in an email that she believes “his approach to mathematical models to be sensible and sound.” Ajaz Hussain, a former deputy director of the Office of Pharmaceutical Science at the FDA’s Center for Drug Evaluation and Research, watched a video describing SoCal’s precision medicine approach and found it “very attractive.” Its use of demographic and individual measurements to characterize the immune response to food allergens and derive a sequential protocol to alleviate the problem, is likely “the methodology for the future,” Hussain said.
Likewise, many families, even without understanding the algorithms and analytics behind the elaborate treatment schemes, believe Randhawa is onto something — and willingly shell out thousands of dollars to find out. “I know it’s costing us, for sure. But it’s got to be worth it, because it’s your kid,” said Craig Folven, president of a flooring distribution company in Eagan, Minnesota, whose son started TIP last May.
For Greg Neuman, as well, SoCal Food Allergy offered hope for his 4-year-old son. Still, after flying the family out from New Jersey for the intake appointment in October 2019, they decided not to continue. “Something rubbed me the wrong way,” Neuman said of SoCal’s secrecy. “It flies in the face of most of the things that I believe about science. You know, if someone was sitting on a cure for cancer or Alzheimer’s and wasn’t sharing it, I’d be very suspicious.”
Indeed, in interviews with Undark, numerous allergists expressed concern about the SoCal clinic’s approach, yet many hesitated to go on record criticizing a method that has not been published in a reputable, peer-reviewed journal. Some details about Tolerance Induction Program methods are described in Randhawa’s 2019 paper in the Journal of Allergy and Therapy, whose content is freely available. However, some such open-access journals have a dubious perception among researchers as pay-to-publish scams.
For Matt Bell, an allergist in Fayetteville, Arkansas, that was the first red flag. “I’ve never heard of that journal before,” he said. “If your data is that great, why did you go that route?”
Another head-scratcher: Unlike typical food immunotherapy studies, TIP patients in this study did not have to undergo a food challenge to confirm their allergies at the start of treatment. “It’s easy to overdiagnose,” said Brian Schroer, director of allergy and immunology at Akron Children’s Hospital in Ohio. Without mandatory challenges, he said it’s possible for some patients to finish the program “‘free eating’ simply because they never were allergic.”
The published study’s retrospective design and enigmatic entry criteria left more room for suspicion. The abstract summarizes “a descriptive study in 51 peanut-allergic children” whose molecular markers were assessed in skin and blood tests conducted before undergoing TIP and one year later. As the paper acknowledged, there was no placebo group. “They picked 51 people, and they’ve treated thousands,” Bell said. “To me, it seemed a little bit cherry-picked,” Bell added.
“Is that representative of the whole group?” he asked.
According to Randhawa, the patients were not cherry-picked. The institute’s algorithms group patients into five endotypes, or subtypes, according to levels of antibody responses to various plant proteins, as determined by analyzing blood samples. The paper analyzed those 51 patients because they fell into the same endotype, meaning they shared a “reproducible set of signals” from the program’s data analytics and received treatment over a specified 30-month timeframe, Randhawa wrote in an email. Yet “the journals would not allow a definition of endotype since it is based on mathematical criteria of biomarkers.”
As for food challenges, study patients were offered them, but none wanted to proceed “given their severe history,” Randhawa wrote. He noted that many OIT clinics also do not routinely food-challenge patients. SoCal Food Allergy does “an objective intake history” and only treats patients whose intake history and molecular markers are consistent with anaphylactic risk, he said.
Randhawa said he has received “way more than” a half dozen rejections from more well-regarded allergy journals when trying to publish this paper and others.
Amanda Lee, a homeschooling mom in Concord, California whose 12-year-old son went through TIP treatment for a peanut allergy, said that when he started in 2013, she was told the method “didn’t have enough patients at the time to make the statistics work for publishing.” Randhawa’s goal was to reach a thousand patients, Lee said. “He and I talked about publications a couple of times about two or three years into the program, and he was all super excited and I totally expected them to come out within a year.”
The fact that the method was unpublished when her son did the program “doesn’t bother me,” Lee said. “I’ve seen lots of people have tremendous success with it.” Still, Lee withdrew her son in 2018 due to uncontrolled allergic reactions during the maintenance phase, when patients consume their allergens on a less frequent basis to sustain tolerance. In the last four years, some 100 patients have dropped out for various reasons, including financial and personal, according to an email from Mega Jewell, who was the head of public affairs at the Southern California Food Allergy Institute at the time of the email. Without publishing studies in a peer-reviewed journal, “not only can you not prove [the method] works, you can’t disprove it,” said Schroer.

The foundation of science is peer review, Hussain said. If the institute continues to use a non-validated method, “they are potentially going to damage an evolving methodology.” Because SoCal Food Allergy’s methods are mathematical, Hussain said, they should be peer-reviewed by experts in artificial intelligence. Once suitable experts have vetted the technical aspects, he said, then physician peer reviewers could assess whether the method works as a food allergy treatment.
Randhawa has presented or co-authored in more than 100 academic conferences, peer-reviewed publications, and educational lectures since 2005, according to his CV. And he published more than 20 papers between 2009 and 2020 — mostly on asthma, cystic fibrosis, and other diseases besides food allergy, including those studied at the research center Randhawa established in 2015.
Randhawa has had a harder time publishing food allergy research. When plant protein databases and statistical “mixed-model” analytics come into play, as they do in SoCal Food Allergy’s treatment approach, “the system does not quite know how to handle it,” he said.
“The reviewers will say the same thing. They’ll say, ‘Well, we don’t understand this concept. We don’t understand the analytics,’” Randhawa said. Editors have suggested submitting the work to a mathematics or statistics journal. They’ve said “there are no reviewers in the field of allergy who are on our editorial board who feel comfortable reviewing this,” according to Randhawa.
Gailen Marshall, Jr., editor-in-chief of the Annals of Allergy, Asthma and Immunology, confirmed that Randhawa submitted two papers to the journal in 2018, both of which were rejected. Marshall did not respond to questions about the grounds for rejection, noting that he was “enforcing an expectation of privacy that all authors who submit to our journal have.”
Once, Randhawa sent the manuscript to editors of a food chemistry journal. But they, too, said “’this sounds too mathematical, and too allergic. We don’t know what to do with it,’” Randhawa said.
“I have a choice, right? I can either continue to play the typical academic game,” Randhawa said, “or I can actually try to treat the patients in an organized, regulated, methodical fashion that follows molecular markers.”
The tension between Randhawa and traditional allergists is nowhere near settled. Several years ago SoCal Food Allergy put out various social media posts and videos with charts comparing TIP’s success and side effect rates with those that had been reported for food immunotherapy trials conducted by the Consortium for Food Allergy Research (CoFAR), a government-funded program established in 2005 by the National Institute of Allergy and Infectious Diseases to support clinical research on food allergies. SoCal Food Allergy’s claims met with skepticism.
“They were touting to be better than anything that CoFAR ever did,” said allergist and immunologist Kari Nadeau, director of the Sean N. Parker Center for Allergy and Asthma Research at Stanford University, one of CoFAR’s seven research sites, and co-author of “The End of Food Allergy.” “We’re like, what? Let’s learn about this. Let’s figure this out. Let’s share it, right? What’s your magic because we want to test it.”
Scott Sicherer, director of one of the CoFAR sites, the Jaffe Food Allergy Institute at the Icahn School of Medicine at Mount Sinai in New York, had a phone conversation with Randhawa in January 2019. Sicherer said he offered to be “an honest broker to test his approach” in about nine Mount Sinai patients per month — perhaps a hundred over the course of a year. The patients would be treated at Mount Sinai according to a personalized TIP plan based on data from skin and blood tests. According to Sicherer, Randhawa said he would present the proposal to SoCal Food Allergy’s board of directors and respond in a few weeks, but that never happened.
In an email message to Undark, Randhawa said that Sicherer was supposed to go back to his own board to obtain funding information. “He never followed up on his end,” Randhawa said. Ultimately, the standoff was moot. Randhawa, who said he recalls Sicherer offering to treat a smaller number of patients in a year, said he had no interest in trying to figure out how to select participants for Mount Sinai’s proposed study given that SoCal Food Allergy’s patient volume is typically much higher.
The two have not communicated since.
Nadeau said she has contacted the Long Beach clinic several times to ask about patients who came to her because they were struggling at SoCal Food Allergy. “I’ve tried to email him just to say, ‘What are you guys doing? Can you please help me help your patients?’ And nothing. Then I tried to call their office. Nothing,” she said. “We need help in taking care of their patients. We don’t know what doses they run. Neither do the patients.”
In a field that requires collaboration to move forward, Illinois allergist Bajowala said it’s hard to understand why “there’s such a veil of secrecy.”
Owing to his other responsibilities, Randhawa says he attends allergy and immunology conferences infrequently. He stays off OIT email lists and has steered clear of collaborations among allergists who collect and publish outcomes and best practices from food allergy immunotherapy in clinical practice. Securing interviews with Randhawa for this story required two months of discussion with the institute’s media contact.
Still, Randhawa estimates that he has spoken to more than a hundred allergists about TIP in one way or another, including through conference presentations and medical school lectures he has given over the past decade. Several physicians from Southern California institutions came to the facility around a decade ago wanting to learn how things are done, he said. And once or twice a month Randhawa receives emails from curious allergists whose patients have come to SoCal. “In the early years, they would just say, ‘Oh he’s just doing OIT,’” Randhawa said. “More recently, they just don’t even know what it is. And they just say, ‘it’s different.’”
These days he’s pushing on another front: getting the institute’s tests and treatment covered by insurance and cleared by the FDA. The number of molecular markers analyzed in patient blood samples has risen from 70 to 80 a decade ago to more than 400 today, exceeding the capability of Quest Diagnostics. That forced SoCal Food Allergy to build its own testing facility.
Foundation Labs, the company Randhawa founded to do SoCal Food Allergy’s testing, got certified by the accreditation organization COLA in 2019 — after massive delays and higher scrutiny stemming from fraudulent blood testing claims that had shut down the Silicon Valley startup Theranos the year before. As of fall 2019, “Foundation Labs is still $3.5 million in debt,” said Randhawa, who says he has purchased millions of dollars of equipment with his own money.
Randhawa said he will continue trying to publish SoCal Food Allergy’s research. But the current system of peer review requires “old forms of analysis,” he said. “I’m not trying to adapt to that. I’m here trying to do something innovative.”
“What you’re really kind of boiling it down to is one word: disruption,” he said. “Am I trying to disrupt the system? Yes.”
Esther Landhuis (@elandhuis) is a California-based science journalist who writes about biomedicine and STEM diversity. Her stories have appeared in Scientific American, NPR, Nature, and Quanta, among other publications.










Comments are automatically closed one year after article publication. Archived comments are below.
This program is the best! Really disappointed in this journalism. My son has been there for a year and is eating things I never thought he would! Keep in mind an allergist has a simple job to make money and if they are asked to do more, it’s considered dangerous and hard. They don’t want to work! So of course they think this is dangerous and what Dr. R is doing isn’t right. None of them care as long as they get their paychecks! A little brain power and dedication will get you to learn more about allergies and how to help save our children and our future! He is great and the other Drs aren’t near his level of smartness. 🤷🏻♀️
Most comments here are so so suspicious.
I was ready to give the place more benefit of the doubt until I saw the most obvious astroturfing campaign ever.
No Undark article gets this many comments, no matter how controversial, and with very similar writing styles and story points.
There is nothing stopping the doctor from publishing his data and getting every allergy sufferer this 99% success rate, if it was true. Nothing that stops it but greed or lies.
Good luck with the FDA.
It works.
This program really works, this article is missing the 10K families that now leave free of fear and children anything they want. My daughter its in the program and it has been life changing. I searched for answers for years and not even the best allergy doctors in NYC could answer my questions as Dr. R did, the men should get a noble prize
We are a patient of Dr R. and can only say positives things about the treatment and Socal Clinic. What people need to understand is that local allergist are virtually clueless – they gave your epi pens and that’s over. It’s even shocking that they allow OIT (try to give a peanut spoon to a kid is insane) – they have no idea whatsover they are doing. My daughter had a strong reaction two years ago to Soy – even if I don’t understand Dr R mathematical model or AI, it does not mean they are wrong. My daughter can know take soy as much she wants and did not experience negative effects. She did it progressively in a control fashion. Same for most of the tree nuts. Is it costly, yes if you don’t live in LA, is it worth it 1000%. Again the folks that are critical did nothing in the last 50 years to improve food freedom – that they are amazed someone did something is may be shocking to them. If anything Dr. R deserve utter respect for saving lives.
I am a physician who had my son treated by Dr Randhawa for severe food allergies to peanuts, tree nuts, soy, seeds and garlic. He was first turned down by an OIT specialist in El Paso because his allergies were too severe. We moved to CA for this program which he completed 2 years ago and he now eats all of his allergens in unlimited amounts. He ate 75 peanuts at his final food challenge. A dusting would have previously sent him into anaphylaxis. Dr Randhawa is a pioneer in treating food allergies and these other allergists you interviewed have no business critiquing what they do not understand. This was an extremely biased and poorly written article. There are thousands of kids and families you could have interviewed if you really wanted the scoop on what is going on at So Cal Food Allergy. Dr Randhawa has saved thousands of lives. Let’s show more respect for the incredible successes he’s had and the countless hours he’s poured into this program.
How many times can one say we use data driven, mathematical models, multiple data points, scientifically driven, without actually publishing or sharing the information. Are families supposed to take your word for it.
I agree. The first thing that came across my mind after reading this article was, this is quack medicine.
My daughter has been in the TIP program here for more than a year, and I was disappointed that this article did not reflect our experience here at all. Before we decided to commit to traveling across the country for this treatment, I did a ton of research and what convinced me was the hundreds if not thousands of parents and young patients that shared their experiences here (on their personal accounts and 1 on 1). Children once anaphylactic to peanut are now eating 75 peanuts – eating freely and safely. I understand that the commitment it takes may not be for everyone (no one said it was easy, but wow it’s been worth it for us) but I thought this article should’ve at least included the view points of families in remission and currently still in the program. I understand that the medical community looks at things differently but why shouldn’t it start with the patients? We have already seen life changing results in this program, and we will tirelessly advocate for Dr. Randhawa and the (safe) expansion of this program in the future.
If the comments here are from actual patients, I can appreciate the frustration you must be feeling having read this article. But speaking as an MD and a scientist, I feel very uneasy with the mystery in which this treatment is shrouded. The math and physiology should be vetted and, if this is really an effective treatment, as the comments here suggest, more clinics should be opened! A 2.5 year waiting list for what may be an effective treatment!? THAT is the most criminal aspect of what I have read here.
The process of desensitization is lengthy and requires very specialized know how. If not done properly kids could experience anaphylaxis and death. Dr Randhawa has greatly expanded his team over the years but it requires careful training. It’s not so simple. I am a physician myself and my 9yo so is a graduate of this program. I’d be happy to demystify the process for you. Finding this program was life changing for our whole family.
Please consider sending your food allergy child or patients to a provider listed on FASTOIT.org like we did. They, like most scientists, strongly believe that learning together, sharing and publishing data and methods for food desensitization will lead to safer, better patient outcomes for all. Shouldn’t all patients, families, and physicians want a better future for all food allergy patients?
This article is not accurate and worst of all it is dangerous for many children whose parents might rethink SOCAL’s life saving treatment because of the author’s irresponsible journalism. As a parent who took the time to interview many allergists throughout the country before meeting Dr. R (a number of which are noted in your article), I am shocked that you are not more careful with your references. Many of those allergists treat their patients as lab experiments, and take huge risks with no reward. So many of the children in other programs end up sicker than before or in the hospital. SOCAL is the only treatment that is safe and effective. The only treatment with low to no risk and huge outcome. Dr. R is so far ahead in what he and his team are doing that he is leaving these other doctors in the dust. Please stop risking the lives of children with your inaccurate journalism.
For those who are interested in the truth, see Dr. R’s response to this inaccurate portrayal of TIP and SOCAL
https://www.socalfoodallergy.org/newsroom?fbclid=IwAR1l1gxirw8mmKsqj6QOdZOvDnPygPdY7_NpYibqjpLonxqcIh4b_aeAJfU
Well said!
Thank you! This article wasn’t great at all.
The only thing “unorthodox” about Dr R’s treatment is that it cures food allergy. The standard of care for everyone else is avoidance and carrying around an epi pen living in fear. SCFAI does sound too good to be true. But what if it is?
Sad that the interviewer didn’t ask the thousands of families living without fear of food to chime in. My daughter is living proof, the program works!
The author didn’t bother to interview even one family who had this “stunning” success with the program. How is this journalism? What a squandered opportunity. She had the opportunity to highlight – and perhaps provide informed critique – of medical advances designed to save lives. Instead, she wrote a hit piece on a methodology she never bothered to investigate. Interview the families that no longer live in terror of death thanks to SCFAI. Interview current participants who see tolerances building in the children with every dose. Do the hard work of fact-gathering and journalism – not the hearsay nonsense published here. This “author” is nothing more than a mouthpiece for the ancient allergists with whom she buddied up to secure sour grapes quotes. Exploiting SCFAI’s “competition” to undermine its unparalleled scientific achievement only highlights the fact that the methodology is too intellectually advanced for this author (and her antique allergist friends) to grasp. Stick to writing Instagram posts, Ms. Landhuis. You bit off more coconut and pumpkin seeds than you can chew.
How would the author have gone about identifying patients to interview? Only by one source: those provided to the author by Dr. R.
The author should be fair & write a follow-up article to provide a more balance perspective. The author could easily contact the TIP parents in the comment section since an email address is needed to post here. There are plenty of TIP patients who have failed OIT and oral food challenges with other allergists but are now thriving & freely eating their previous allergens after being treated by Dr. Randhawa. These patient families need to be interviewed.
Hi Seth, maybe I’m missing something but I found all my patient sources on social media. It’s easy when you look for the hashtags. Could she not have reached out to patients that way?
Dr. Randhawa is prohibited by law from releasing patient names. However, it is the hallmark of journalism to locate sources for a story. Is it your position that we should spoon-feed journalists their sources and stories? How quaint. The author could have easily searched any social media platform and found a plethora of families who actively contribute to several SCFAI-based groups. As one of them, I can assure you I would have been happy to discuss our experience in the program. It is not rocket science to conduct journalistic research. Which is why this piece should not be hawked as reliable journalism. Owning a keyboard doesn’t make you a writer. I hope this author will reconsider the dangerous condition she is creating by falsely redirecting the trust of prospective families away from the program.
What kind of journalist only shows one side of the story? This is quite amateurish. We are patients with Dr. Randhawa and in remission thanks to him. No other allergist wants to spend the time or money to treat patients like Dr. Randhawa does. We haven’t been able to go anywhere or do anything because of our allergic daughter. Years of being terrified of an allergic reaction. She is free eating now, can eat as much or as little of her allergens as she wants with no reaction at all. I’m forever grateful to Dr. Randhawa.
I need to agree with the article. 10,000 patients treated, that’s just a huge number when compared to a successful OIT clinic that is busy at 500 treated.
The math model that predicts their exact dose is suspicious.
Plenty of individuals compare of reactions into their Facebook group, which they delete those comments. Plenty of allergists see their patients and can tell either 1) they informed somebody that they were anaphylactic when they were not or 2) do bill patients to over challenge foods that the patient was already likely not allergic to
It’s known if you are allergic to peanuts you are likely to have + results to lentils, peas, chick peas, soy etc etc etc most of these could be home introductions without having to challenge each individual food.
Lastly it’s very telling that they don’t hire allergists. They have this air, and inform their patients that allergists are “too dumb” to understand their approach. Somehow someone with no training in food allergies prior to working there can be an expert tho.
The approach of treating cross reactive foods has promise, but this company covers it with over selling claims, hyped up marketing, and overzealous fans.
From what I understand, in the past, other allergists were more interested in stealing the Southern California Food Allergy Institute’s wealth of data rather than making a long-term commitment to actually working at the clinic.
Thank you for your article. My son is one of many in remission from tree nut allergies. Please share both sides of the story, because this article is very biased. Our experience was smooth, with zero reactions, and took one year to complete. He graduated in January 2020 and continues to eat absolutely everything with no issues whatsoever.
My son is 19 and one year into remission after being treated at SCFAI. Thanks to this treatment, he is freely eating all of his allergens. This treatment is real, it is life changing and life saving.
Thank you for sharing information about the SoCal Food Allergy Institute. My 8 year old daughter who is allergic to dairy, eggs and peanuts is a current patient. Prior to TIP she had airborne reactions to eggs and peanuts and an anaphylactic reaction to drinking a sip of milk. We have been in active treatment since Feb 2019 and she is able to eat an entire cooked egg (or more if she wants) everyday as of December 2020! She dyed Easter eggs for the first time this year! She is still working on dairy and peanuts, but so far the process has been smooth and we are hoping for the goal of food freedom by the time she’s 10!
There are 10,000+ who will vouch for the success of Dr. Randhawa’s program, myself included. The results speak for themselves.
Wow, what a roller coaster of emotions this article causes. It was like 2 different persuasive essays with a calm, factual mediator in the middle – Dr. Randhawa being the voice of reason. Of course people are questioning all of this, I did too. It sounded too good to be true, but after listening to his logic/science and actually witnessing it working as well as seeing his insane work ethic, I could not even think about going back to “avoid and carry an epi” or try another method that just doesn’t feel as safe. I think this article was fair but not completely balanced because it seems that more people that left the program or never even joined in the first place were given the amplifiers. At least Dr. R was given the opportunity to clearly articulate why publishing has been so difficult and why there was a need to create their own lab. There are hundreds of us that would be ready and willing to share our experiences (and yes, my son was really allergic in the first place…I can’t believe people would insinuate that he treats people that aren’t even allergic.)
It’s true- there is no place like SoCal Food Allergy Clinic. The one and only place where the doctors truly take the time to study your child’s allergic history and immune system to create an individualized program that does not involve a drug or a patch. For those of you thinking, “He’s on to something” – you’re right! ❤️
My son is a patient at SoCal Food Allergy. I can help clear up info and misconceptions I feel you may have after reading your article.
Curious why no patients who successfully completed treatment at SOCAL and are now in remission were interviewed for this story? My 19 year old son completed treatment at SOCAL in January 2021. This program has changed his life!
Thanks for taking the time to document this
Dr. Landhuis. we r open to discuss our journey from airborne reactions to peanut & cashew dust to passing 75 peanut challenges and our rocky history with dairy Dairy OIT & slowmo TIP anytime! always here to help others in our shoes