For decades, the treatment of clinical depression has followed the same outdated flow chart: Try one antidepressant, then another, then a combination, then maybe just one more. Finally, when all pharmaceutical attempts fail, consider other approaches. This protocol, called step therapy, has come under tremendous scrutiny, as it prioritizes the least expensive treatments over ones that may be more expensive but also more effective. This practice serves to protect insurance companies’ bottom line, potentially at the expense of patients’ well-being.
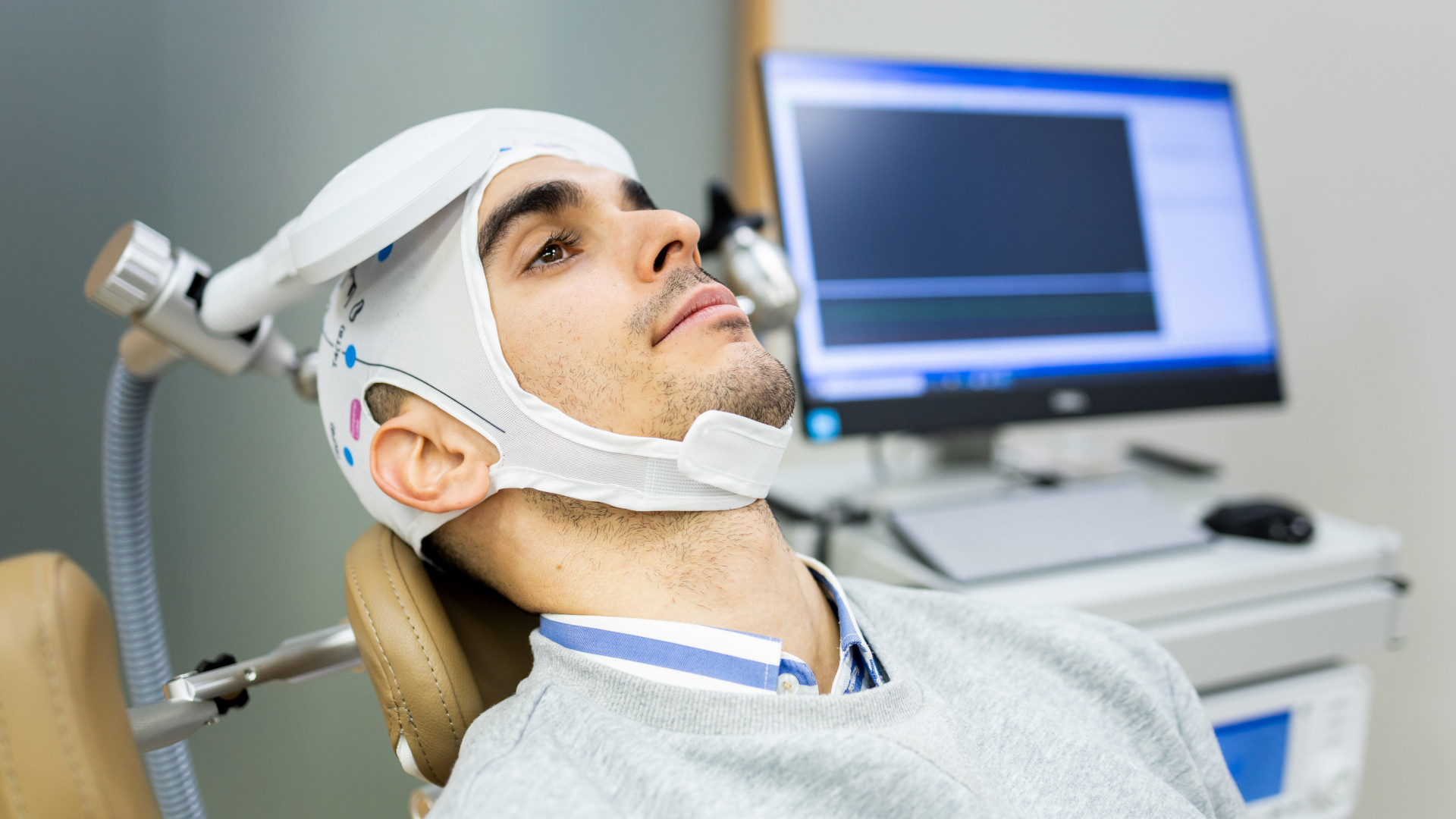
One such treatment is transcranial magnetic stimulation, or TMS, which was approved by the Food and Drug Administration in 2008 for adults with major depressive disorder, also known as MDD. Since then, clinical research has only strengthened the case for its safety and effectiveness. Yet insurance rules still generally restrict coverage to patients who have already endured multiple unsuccessful medication trials. These outdated policies keep patients trapped in cycles of trial and error, which are time-consuming, demoralizing, and often medically unnecessary.
TMS does not use medication but magnets. A highly specialized piece of equipment containing an electromagnetic coil is placed above a region of the brain known to be functionally underactive in clinical depression, and delivers rapid magnetic pulses to stimulate brain cells in the area. The magnetic field induces small electrical currents that alter activity patterns of cell populations and strengthen their connections. This modulation helps restore normal communication within mood-regulation networks with no anesthesia, no systemic drug exposure, and no demonstrated lasting adverse effects.
One drawback: TMS can be a slow process. When used in psychiatric treatment, it typically requires patients to undergo 20 to 36 sessions, each lasting an average of 30 minutes, over four to six weeks. This, however, can be seen as front-loading the time commitment that depression generally steals from a patient. Instead of losing time — sometimes years or decades — to managing symptoms, the effort is concentrated into a relatively short, structured timeframe with a clear endpoint.
These outdated policies keep patients trapped in cycles of trial and error, which are time-consuming, demoralizing, and often medically unnecessary.
The therapy is also harder to access than first-line treatments like antidepressants, since it requires a special device, though it’s still offered in many settings, whether a psychiatrist’s office, a hospital, or a specialized clinic. The maker of NeuroStar, a widely used TMS device, reports that its machines are available in more than 1,100 locations in the U.S. alone.
TMS is generally very well tolerated, with an excellent safety profile and no systemic interactions with medications. The most common side effect is mild, temporary scalp discomfort or headache caused by muscle contractions around the targeted area. The primary serious risk is seizures; however, they are rare, occurring an estimated 1 out of every 30,000 sessions. Nevertheless, a physician does need to oversee the treatment and be available during the sessions, which is helpful in the event of a seizure.
Compared with other first-line depression treatments such as selective serotonin reuptake inhibitors, or SSRIs, TMS poses fewer risks. SSRIs like Zoloft and Lexapro, while often effective in treating MDD, come with a host of potential side effects: Weight loss or gain, sexual dysfunction, gastrointestinal distress, and withdrawal symptoms upon discontinuation are all relatively common.
Most importantly, clinical data show that TMS can be effective in treating MDD. In a 2023 review published in BMC Psychiatry, researchers analyzed a total of 19 double-blinded, placebo-controlled studies, nine of which looked at remission after treatment with TMS. While the trials differed slightly in methodology and stimulation parameters, they all asked the same core question: Does TMS help adults with major depressive disorder, specifically those who had already failed two different drugs? (While I argue that the lack of success pharmacologically should not be a prerequisite for TMS treatment, drug resistance still serves as a good gauge for depression severity in clinical research.)
The researchers found that TMS led to significant reductions in depressive symptoms when compared to placebo stimulation. Additionally, the therapy also showed a higher likelihood of full remission, not just symptom reduction. The authors concluded that TMS is not only clinically effective but also well-tolerated. They further noted that across several meta-analyses, the magnitude of benefit was comparable to that of pharmacotherapy, which suggests it could be a legitimate first-line treatment rather than a last resort.
Today, novel research is still being conducted, further demonstrating safety and efficacy. A 2025 study out of Australia, for example, showed that teens and young adults with MDD responded well, similar to previously established rates found in older adults.
Depression already steals time from people’s lives. An insurance policy shouldn’t take up more of it.
But despite its scientific backing, patients are often required to try multiple medications before being eligible for reimbursement for TMS because of the widespread adoption of step therapy by insurance companies. This leaves patients spending months or even years in ineffective medication cycles, which can worsen depression severity and drive up long-term costs.
Depression already steals time from people’s lives. An insurance policy shouldn’t take up more of it. The science behind TMS has advanced far beyond the regulatory framework that governs its use; it’s time for insurance coverage to reflect this advancement. Patients shouldn’t have to fail twice before being offered a treatment that works differently, safely, and sometimes more effectively than medication alone. If TMS therapy could be covered by insurance initially, then it could be presented alongside first-line medications as a potential option, allowing for full, informed patient consent.
Clinicians should advocate for early access to TMS, and insurers should revise step-therapy rules to treat it as a first-line option. The FDA approved TMS nearly two decades ago, and the research since has only reinforced that decision. The data are precise; what’s outdated isn’t the science but the policy that keeps people from benefiting from it.
Michael C. Marone is a neuroscience graduate student at Georgetown University’s School of Medicine and a freelance science writer.