Interview: How a Hearing-Loss Grant Got Cut in the Fight Over DEI
A few years ago, the National Institute on Deafness and Other Communication Disorders issued a call for proposals aimed specifically at researchers from diverse backgrounds. The funding opportunity seemed like a good fit, said Uri Manor, a scientist at the University of California San Diego who qualified for the award because he studies the biology of the inner ear and has a disability, severe-to-profound hearing loss. Manor applied, and in 2023 received a prestigious five-year grant.
In its first two years, the grant provided about $1.5 million, which Manor’s lab used to develop new imaging tools and to conduct an array of experiments to better understand — and eventually treat — hearing loss. A third year of grant funding was supposed to kick in on April 1. But so far, that money has not arrived. Instead, Manor received an email informing him that the grant had been terminated in part of the Trump administration’s broad effort to root out DEI initiatives — an experience Manor recently made public in a long thread on X.

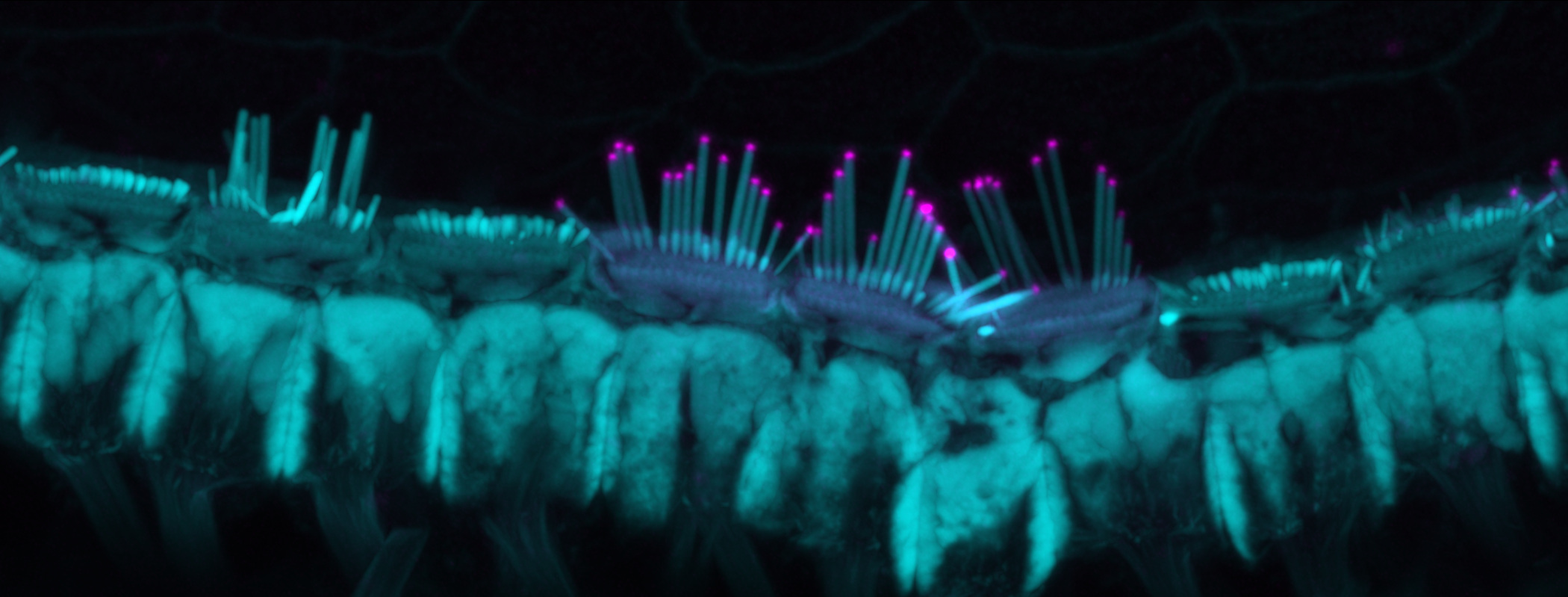
Uri Manor studies the biology of the inner ear, and also has severe-to-profound hearing loss. A federal program aimed at increasing workforce diversity awarded him a five-year grant in 2023, but this spring the funding was cancelled.
Visual: The Salk Institute for Biological Studies
“It felt like the floor dropped out,” he wrote in an email to Undark. His lab employs a dozen full-time people, and he worried about the four dedicated trainees whose salaries the grant was supposed to fund and “whose careers now hung on a political whim.”
A judge recently ruled that many of the DEI-related grant cancellations were illegal. On Monday, Manor’s grant status was updated to “pending,” though no explanation was given. (NIDCD’s parent agency, the National Institutes of Health, did not reply to a request for comment.) The money is not yet in hand, however, and Manor has started the process of laying off trainees, winding down experiments, and writing new grant proposals.
On Tuesday, Manor spoke with Undark about his interest in specialized cells of the inner ear, about the grant termination, and about the return on investment to U.S. taxpayers when they support biomedical research. The interview, conducted over Zoom with follow-up by email, has been edited for length and clarity.
Undark: This grant funded research into hearing loss. Can you talk about the public health impact of this condition?
Uri Manor: Almost everyone has someone in their life with hearing loss — if nothing else, their grandparents or even their parents, but also military veterans, farmers, factory workers. And also one in 400 children like me are born with severe or profound hearing loss.
A lot of people don’t know that it’s also the number one modifiable risk factor for dementia. If you have hearing loss, you have a two to three hundred percent increased risk for dementia, which a lot of health economists are predicting is going to be the number one public health expense facing society as we age. Some are predicting it’s going to cost trillions of dollars. So being able to treat or slow down hearing loss is a huge public health concern.
UD: You’ve been studying something called hair cells, which are found in the inner ear. What do these do, and what specifically is your team hoping to understand about how they function?
UM: Hair cells are, in my opinion, the most amazing structures in the biological kingdom. Most people know that there are these little snail shells that live inside our heads, those little spiral bones that look like snails [called the cochlea].
Inside that shell is a sensory tissue that if you unwrap it into a long line, you’ll see that it’s like a string that’s really stiff on one end and really loose on the other end. That allows different frequencies to resonate at different positions along that spiral. And if you look closely at that string, you’ll see that, actually, there’s a row of strings on top of that string — like a harp, where there’s really long strings on one end and really short strings on the other. Those are the little hairs on the so-called hair cells. They’re called stereocilia.
We’re really interested in understanding how those hairs can function for a lifetime. And we know that as you get older, they can become disrupted, and they can become damaged. And we also know that a lot of deafness mutations cause them to not grow to their proper shape or their proper length.
For my Ph.D., I identified one of the key proteins involved in making sure that they grow to the proper length. It’s called EPS8. That’s what I’ve been studying ever since, and that’s what my grant was on: trying to understand how EPS8 promotes the elongation of stereocilia and trying to develop therapies to regrow stereocilia in people who are born with short stereocilia.
Whenever [stereocilia] vibrate, that causes the synapse at the bottom of the cell to fire, and that sends an electrical signal back to the brain. That’s also something that degenerates with age, and that’s another thing that my lab has been studying. We’ve been trying to find ways to regenerate those synapses.
UD: You personally were born with severe to profound hearing loss. Has this informed your research in any way?
UM: I didn’t grow up thinking I was going to study hearing loss — I actually studied physics. But then, when I saw these images of these hair cells, I sort of fell in love. And at the same time in my mind, I had like a goose-bump experience: Maybe it’s my destiny to study hearing loss, as someone with hearing loss. There was a sense of purpose.
I have to say that, also, growing up with hearing loss gave me a lot of skills that have really helped me as a scientist. I don’t hear any consonants. I only hear vowels. And I also have severe ADHD, by the way, which is a pretty bad combo for someone who needs to focus to understand what people are saying. So, I only receive a small fraction of the information conveyed to me in speech.
Throughout life, I have had to learn how to create and infer patterns to understand what people are saying in real time with limited information. I’ve become a very big pattern-detector and interpolator, which, by the way, is what a lot of the deep-learning AI tools that we build in my lab do, also. I think that’s one way growing up with hearing loss has helped me develop my brain, like kind of a superpower.
Also, famously, if you have a deficiency in one sense, often you grow strengths in other senses. So I have a very good visual sense, which served me well as an imaging scientist and microscopist.
And then I did an undergrad summer research internship through the AAAS Entry Point! program, which pairs students with disabilities with summer research internships. That’s when I actually learned about the NIH. I ended up doing my Ph.D. at the NIH. I think those kinds of programs really show that when you give talented people who have faced hurdles in their life a fair shot, sometimes they can out-hustle and out-innovate the rest.
UD: What was your first thought when you received the termination letter?
UM: The first thing I thought of was my people. What’s going to happen to my people? I have a nine-month salary. I can teach. I have other funding. I’m still going to have my job. It’s the people who I was really scared and worried about, and, of course, the science.
I went into the craziest grant-writing spree of my life, where I wrote five [grant proposals] in a period of about three weeks. I slept maybe two hours a night while also keeping a strong face and reassuring my team not to be scared, that we’re going to figure out a way.
UD: This may seem like a broad question, but it seems relevant. Why should U.S. taxpayers fund biomedical research? And have we been getting a good return on investment?
UM: I actually think this is the most important question to discuss right now.
You’ll see a lot of articles about how awesome science is and how great it is, and how hard it is to lose this funding. I’m certainly participating in that, talking about how difficult it is. But I think there’s another really important message that gets lost, which is that we’re very lucky to have taxpayer funding. It’s a privilege, and that means that it comes with a responsibility that we have to do good with it.
I also think, on the other side, there’s a misconception about government-funded workers or scientists. I think a lot of the public may not know just how insane we scientists are, that we work 80-, 90-hour weeks because we’re so passionate. Our dream is to come up with a cure for diseases or a new insight into the mechanisms of life that then leads to hundreds of cures.
That’s our dream, and we’re driven by that more than anything else. There’s a lot of biotech and pharma jobs out there that pay twice as much for half the work. I and people in my lab have opted to be part of the discovery engine here and really try to find something new. The point is that it’s hard to get a better return on your investment than that.
People don’t realize this also: You have to write about 20 grants to get one because it’s so competitive. The people who do get to do this are the best of the best. I think that’s a pretty good return on investment.
For the record, I have visited big pharma labs. When I go there, my jaw hits the floor. I’m in awe of the resources they have and what they’re capable of. These engines are capable of producing real drugs that go into real patients, and that’s a role for industry and the private sector. But they also need us. Those big companies have to respond to quarterly reports to investors, and they’re like giant battleships that can do certain things really well. But they’re not nimble, they’re not versatile. They need us to do these crazy high-risk, high-reward experiments that can then inform them.
As far as my own grant and return on investment: I think it’s fair to ask the question, what about my grant?
UD: Yeah, what about your grant?
UM: What about my grant? Was there anything good that came out of that? I would like to say “yes.”
One thing we did was — I mentioned age-related hearing loss — we figured out that a pretty popular, common vitamin, vitamin B3, can slow down age-related hearing loss. I mentioned before that hearing loss is correlated with dementia. We have a really nice paper with collaborators showing that actually, it’s that NAD pathway that is strongly correlated in Alzheimer’s disease and age-related hearing loss.
UD: As a researcher, what would you hope our scientific leaders are thinking about right now?
UM: I hope — and I think they are — they’re thinking about how to reward high-risk, high-impact research in academia, real discovery science. I think that we need to be thinking more about how to build, or rebuild, or restore, trust. And more transparency: I think open data.
There’s a lot of talk about people being required to share their data, but we need to make it easy, especially now with AI. My fantasy is that someday we don’t even publish in journals. We just publish every experimental data in real time into a giant server that can crunch all of the data, and we can have a ChatGPT for biology. So it’d be nice to incentivize more open sharing.
And less competition and more collaboration. We’ve sort of plucked away all the low-hanging fruit, and now the next frontier, I think, is going to be big team science and more collaboration, interdisciplinary collaborations. More funding stability is required for that. These short one-year, two-year, five-year grants may not be enough. We may need more like seven- or 10-year funding opportunities to really do the best work we could possibly do.
I think some of the changes at the NIH that are being proposed or implemented are in line with that. I think that some of the things they’re trying to do are good: simplifying the review process, incentivizing high-risk, high-reward [research]. I think that there’s a lot of good happening there too, and we need to acknowledge that.
UD: Is there anything else that you’d like to add?
UM: I would just want to emphasize how important what I said about the taxpayer dollars and the gratitude that we have, the privilege that we have and that I think we are doing our part in making this a really good return on investment.
Science should not be a political beach ball. We all want life-saving research to happen. I think we all want the U.S. to be in charge of that. I don’t think we want to end up some day having to pay some other country for drugs and discoveries that they’re making. We should try to keep it here in the U.S.








Undark has a way of making even the most complex topics accessible and engaging. I’m constantly impressed.