Brushing with Bacteria: The Debate Over a GMO Tooth Microbe
About seven years ago, Aaron Silverbook and his then-girlfriend, a biologist, were perusing old scientific literature online. “A romantic evening,” joked Silverbook. That night, he came across a study from 2000 that surprised him. Scientists had genetically engineered an oral bacterium that they said could possibly prevent tooth decay: “I read it and sort of boggled at it and said, ‘Wow, this is a cavity vaccine. Why don’t we have this?’”
So, Silverbook tracked down the primary author, Jeffrey Hillman, a now-retired oral biologist formerly at the University of Florida, to see if he could pick up the torch.
In 2023, Silverbook founded Lantern Bioworks, which made a deal with Oragenics, the company Hillman co-founded and that owned the technology, for the materials. Lantern Bioworks then launched the genetically engineered bacteria under the name Lumina Probiotic. “I didn’t expect it to happen in my lifetime,” said Hillman.

About seven years ago, Aaron Silverbook came across a study of a genetically engineered oral bacteria that he described as a “cavity vaccine.” Now, his company is launching the bacteria as a one-time home application product called Lumina Probiotic.
Visual: Courtesy of Aaron Silberbook
As recently as last month, a website for the product included language about cavity prevention. And a previously available press kit stated that “a one-time brushing with this genetically modified bacteria could indefinitely prevent dental cavities.” By the time Lumina became available for pre-orders last week, however, that wording and the press kit had been removed. Silverbook — who does not have a background in dentistry or microbiology — told Undark that his lawyer advised the change in wording on the website, as Lantern Bioworks is bringing the product to market as a cosmetic, meaning it can’t make health claims about Lumina. Cosmetics don’t need to go through the same rigorous trials a drug would. “If anything I said sounded like a medical claim,” Silverbook told Undark in an interview earlier this year, “it wasn’t.”
The product can be applied to teeth as a one-time application either at home or by a dentist. Additional applications can “expedite inoculation,” Silverbook wrote in a follow-up email. He said the company anticipates Lumina will ship by mid-June.
Some people have already received it. Silverbook said he introduced Lumina into his own mouth in October of 2023, and that Lantern Bioworks has also provided it to about 60 people, including attendees of Vitalia, a biotechnology conference held in Honduras earlier this year. At the conference, Lumina was offered for $20,000 per treatment, though the pre-order price has been reduced to $250 before taxes and shipping fees. (Silverbook would not comment on how many people went for Lumina at the conference.)
Experts, though, have safety and ethical concerns: Despite earlier efforts by Oragenics, the treatment has never successfully moved through human clinical trials. “Without human trials, you really can’t determine whether it’s safe or efficacious,” said Jennifer Kuzma, a professor and co-director of the Genetic Engineering and Society Center at North Carolina State University. In fact, it’s possible it could do the opposite of its original intention: She noted that subtle changes in the oral microbiome might lead to more cavities or other problems.
“I read it and sort of boggled at it and said, ‘Wow, this is a cavity vaccine. Why don’t we have this?’”
There’s also no data about whether it could spread between people, which brings up questions of informed consent. If someone doesn’t want to risk taking the untested bacteria, but kisses or shares spoons with someone who got the product, would it be transmitted? No one is quite sure.
Although Lantern Bioworks is bringing Lumina to market as a cosmetic product, precisely how it should be categorized isn’t entirely obvious, Kuzma points out: “The regulatory system isn’t 100 percent clear on this.”
The human mouth contains hundreds of species of bacteria that function together in a community — an oral microbiome. A healthy bacterial balance keeps teeth and gums in good shape.
Cavities are caused by acid-producing bacteria. Several kinds of oral bacteria can make acid, explained Jonathon Baker, an assistant professor of dentistry at Oregon Health & Science University. But one bacterium, Streptococcus mutans, especially wreaks havoc because it can make both acid and biofilms, including dental plaque. That sticky coating traps acid on teeth, eroding tooth enamel and creating cavities.
In the 1980s, Hillman discovered a naturally occurring version of S. mutans that secretes the antibiotic mutacin 1140. Because mutacin broadly kills other species of bacteria, he realized it could potentially outcompete other harmful strains. (It’s not known how many people naturally have mutacin-producing S. mutans in their mouths; Hillman found this version in one sample out of 115.)
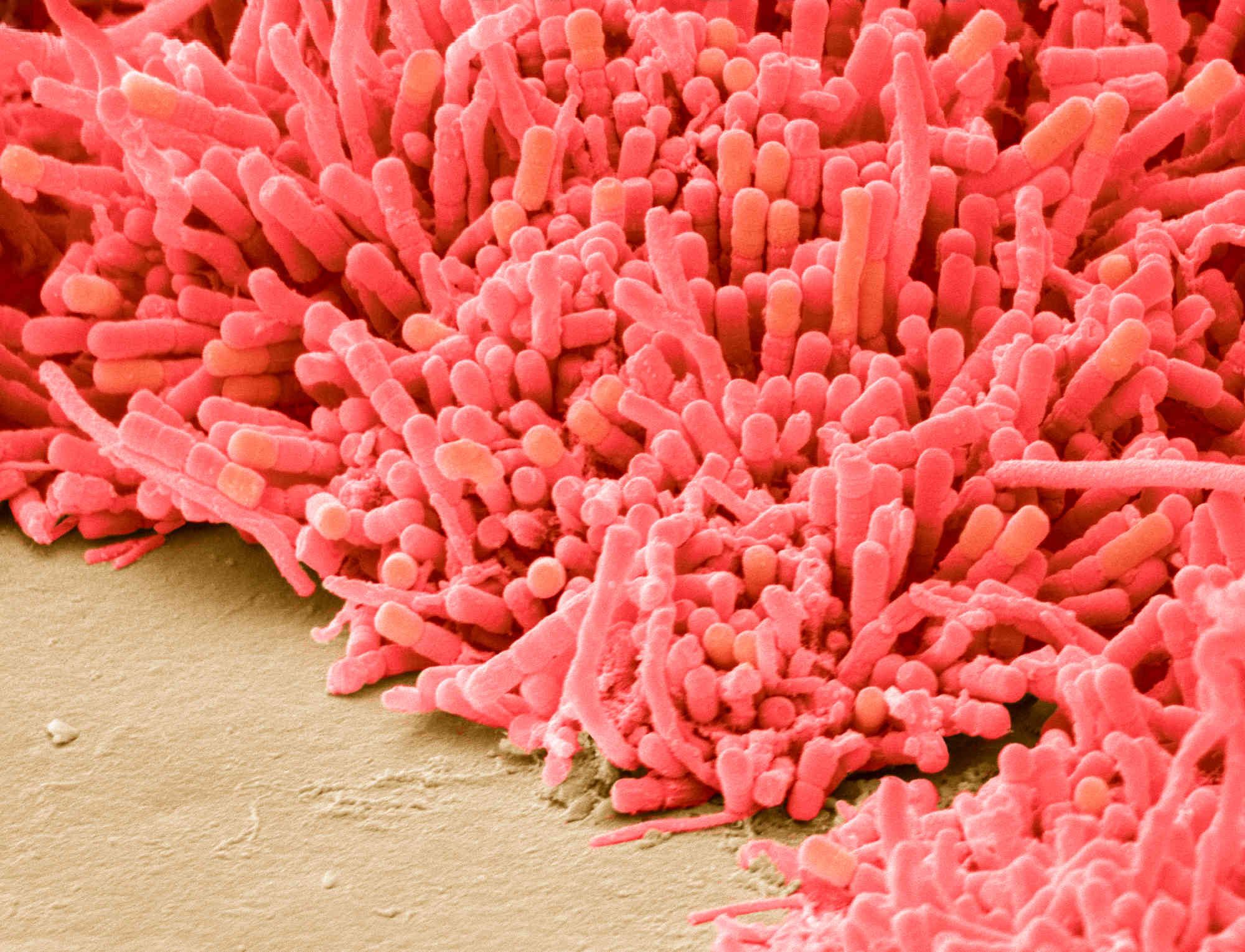
To make sure the bacteria wouldn’t cause cavities itself, Hillman genetically altered it to metabolize sugar in such a way that it produces alcohol instead of tooth-damaging lactic acid, like other cavity-causing Streptococcus strains do. Bacteria often swap genetic material, so he also tweaked the strain to prevent it from taking genes from other bacteria; other bacteria can still take genes from the genetically modified Streptococcus.
Hillman announced the new strain, called BCS3-L1, in 2000. Around that same time, Oragenics sought to begin a clinical trial. But due to concerns that the bacteria could be transmitted between people, have unintended consequences, or revert back to a cavity-causing strain, the company told The New York Times in 2004, the Food and Drug Administration put constraints on the trial, which was then never completed.
Hillman continued his research: A 2009 study showed that his bacteria appeared to colonize rats’ mouths and outcompete other Streptococcus strains that can contribute to tooth decay. But studies have been limited since, and Hillman and Oragenics eventually abandoned the project. About 12 years ago, he retired.
“Without human trials, you really can’t determine whether it’s safe or efficacious.”
Silverbook picked up where Hillman left off: The genetically modified version of Streptococcus mutans is the basis of Lumina. After considering whether to market it as a cosmetic product, as teeth whiteners are, or as a probiotic, which falls under FDA regulation as a dietary supplement, Silverbook decided to go the cosmetic route. In the U.S., only drugs and medical devices must go through the strict process for the FDA to deem them safe and effective.
The FDA did not provide direct comment in response to a list of questions sent by Undark. Courtney Rhodes, an FDA spokesperson, did, however, point to the agency’s website which notes that drugs are defined by their intended use for “diagnosis, cure, mitigation, treatment, or prevention of disease,” and that mismarketing a product is against the law.
Lumina isn’t the first genetically engineered bacteria to hit the market: A company called ZBiotics launched a hangover preventive probiotic in 2019. The small drink contains Bacillus subtilis bacteria, common in other probiotic blends, but with an added gene that helps break down acetaldehyde, a byproduct of alcohol linked to headaches. (There is no published evidence that it helps with hangovers, nor colonizes the gut — there’s only a small toxicity study in rats.)
Lumina is different, though, in that it’s a modified version of what is technically a pathogen that causes dental decay, said Paul Jensen, a biomedical engineering professor who studies oral microbiology at the University of Michigan. Other probiotic supplements are “generally regarded as safe,” Jensen said. “They’re usually in our food to begin with. We know that they’re not pathogens.”
When asked about concerns that distributing a modified bacteria could potentially cause harm, Silverbook responded, “I think that this is not the kind of air-quotes pathogen that will make anything worse.”
Hillman’s goal, was to make “an affordable product that helps prevent a very painful disease,” he said, “but, you want it to be safe and effective.” When asked if more research is needed to determine its safety and effectiveness, he responded “I was certainly always planning to do more studies,” but declined to comment further.
Silverbook sees his product as a way to save people time and money: Dental care costs $136 billion a year in the U.S. according to the Centers for Disease Control and Prevention. Untreated dental infections can also be dangerous, “and there’s plenty of people who, for whatever reason, have a lot of trouble going to the dentist at all,” he said. “It would be really nice to have something that helps with that.”
Silverbook stressed that he is not making a medical claim — a key strategy, he said, in terms of regulation. “A lot of our regulatory system is based on claims, and if you’re really careful about not making a disease treatment claim, then you can get around the drug safety and efficacy trials,” said Kuzma, the North Carolina State University professor.
Clinical trials in the U.S. are especially difficult to carry out, said Jeff Banas, a professor and microbiology researcher at the University of Iowa College of Dentistry, because they are expensive, take time, and face stricter guidelines.
“If you’re really careful about not making a disease treatment claim, then you can get around the drug safety and efficacy trials.”
“If we do a clinical trial, then we are a drug and we cannot sell it unless I have half a billion dollars and 10 years,” Silverbook said.
Scott Aaronson, a theoretical computer scientist and professor at the University of Texas at Austin, volunteered to take Lumina after reading about it on a blog, and got it during a trip to Berkeley, California. He had lost trust in the FDA during the pandemic, such as when the agency delayed the rollout of Covid tests. When he heard that Lumina might help with cavities, Aaronson “was completely ready to believe that if something like this existed, then it would not have been approved by the FDA.”
“I gave this a try simply because it was fast and easy,” he wrote in an email to Undark. “And the downside risk seemed negligible.”
Jensen, the biomedical engineering professor, cautions that more research is needed to show that genetically engineered microbes can improve oral health and don’t have unintended side effects.
The antibiotic in Lumina, he noted, could potentially wipe out other Streptococcus species that are associated with good oral health. And there are other microbes at play that determine gum health. “They’re not the same bacteria that cause tooth decay,” he said. “But you have to be worried about messing up those communities at the same time.”

Lumina Probiotic is applied to the teeth much like a toothpaste, which Lantern Bioworks says inoculates the mouth with a type of bacteria that normally can cause tooth decay. This strain of the bacteria, however, is genetically engineered to avoid making acid and also to produce an antibiotic that kills harmful bacteria.
Visual: Courtesy of Aaron Silberbook
Additionally, people swallow about a liter of saliva every day, meaning the S. mutans’ antibiotic could end up in the intestine, potentially disrupting the gut microbiome, Jensen said. And because Lumina produces alcohol as a byproduct, it’s possible that might have an impact too, though the amounts are tiny and it’s hard to know without more research, he added.
In an email to Undark, Silverbook wrote that the company has been following the “fifty or so” people who have volunteered to take Lumina, and that no adverse events have been noted so far. Their follow-ups, according to Silverbook, consist of a self-reported survey and taking swabs to track whether S. mutans has successfully colonized the mouth. “We haven’t yet decided how long the follow up will be,” Silverbook wrote in another email, “but we anticipate colonization of the mouth to take about two years.” Regarding concerns about the gut microbiome, he wrote that Lumina “isn’t especially intended to be swallowed.”
Given the lack of evidence, Jensen said, “I don’t think I would take it.”
Quite a few oral probiotics have been tested in clinical trials as a solution for better oral health, though none of those are genetically modified. These products aim to add beneficial bacteria to the mouth. For instance, ProBiora, a chewable oral probiotic already available in the U.S., showed a modest reduction in cavities in children in one clinical trial. (ProBiora3, the bacterial blend in the tablets, was developed by Hillman at Oragenics.)
The Lumina microbe, though, was intended to be a kind of oral replacement therapy, so that the genetically modified bacteria would fight and overtake bad bacteria. Replacement therapy, Hillman said, “is doing what nature would eventually do given enough time. Pathogens, especially organisms that live with us on a day-to-day basis, do not want to harm their host. It’s contrary to their own self-interest.”
The antibiotic in Lumina could potentially wipe out other Streptococcus species that are associated with good oral health.
Though the idea isn’t new, no one has made it work yet. Banas said that 10 to 20 years ago, “it seemed like every grant application or manuscript justified their work by saying, well, this can be used for replacement therapy.” But he pointed out that so far, the field hasn’t seen any replacement strategies come to fruition. He’s currently screening thousands of oral microbe strains for potential probiotic properties to develop an oral probiotic for children.
Some experts are skeptical that introducing bacteria could successfully colonize adult mouths. Once the oral microbiome is established, it’s hard to change, said Isamar Rivera-Ramos, a pediatric dentist and dentistry professor at the University of Rochester — though not impossible.
Indeed, Hillman said it’s not easy to introduce new bacteria into someone’s mouth — his team was “uniformly unsuccessful” until they found the naturally occurring strain that makes mutacin 1140. Decades ago, Hillman introduced that strain into his own mouth, and he said it was still there the last time he checked before he retired. He added, though, that he never put the genetically modified version in his mouth, or anyone else’s.
Jensen, Baker, and Kuzma also expressed concern about the potential for the bacteria to be transmitted between adults, as well as children, especially given the lack of relevant data. People acquire their oral microbiome as children from caregivers and other close contacts, so if a new microbe could colonize an adult’s mouth, it could spread to their child.
“If you are pregnant, breastfeeding, or expecting to become pregnant, consider carefully before sticking our magic tooth sauce onto your teeth.”
Baker said studies show that microbes transfer within families and cohabitating people, and noted that “it’s definitely possible” for Lumina to spread. And according to Jensen, S. mutans has “been passed from human to human since it became a species. So it’s obviously very good at moving between humans; otherwise, it’d be an evolutionary dead end.” Hillman, though, noted that introducing microbes for oral replacement requires 100 to 1,000 times more than would be exchanged in normal interactions.
Silverbook told Undark that he is not overly concerned about the raised safety and ethical concerns, though he acknowledged some risks, such as the likelihood for it to spread to children: “If you are pregnant, breastfeeding, or expecting to become pregnant, consider carefully before sticking our magic tooth sauce onto your teeth.”
Christina Szalinski is a freelance science writer with a Ph.D. in cell biology based near Philadelphia.