Drug-Resistant Malaria Is Emerging in Africa. Is the World Ready?
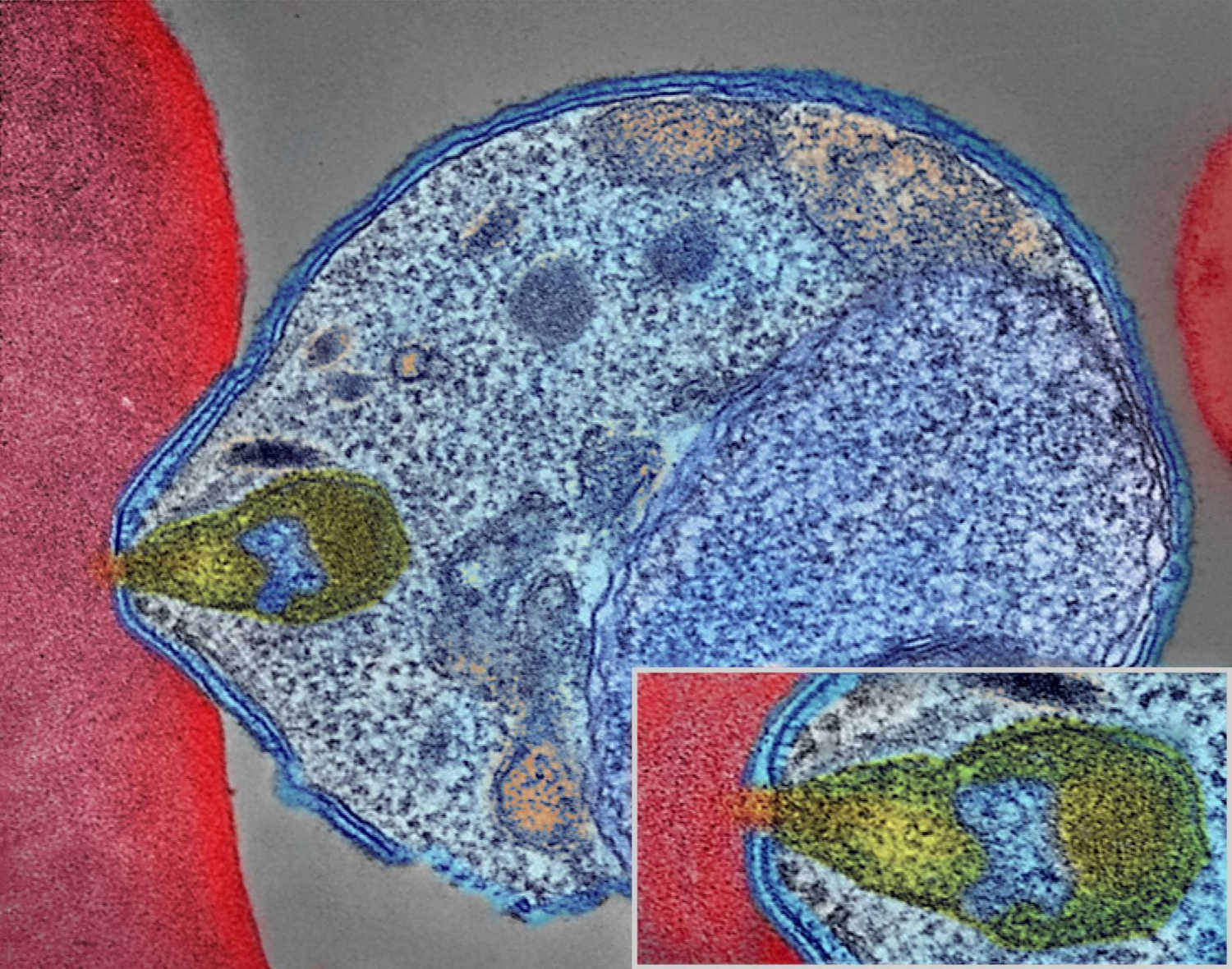
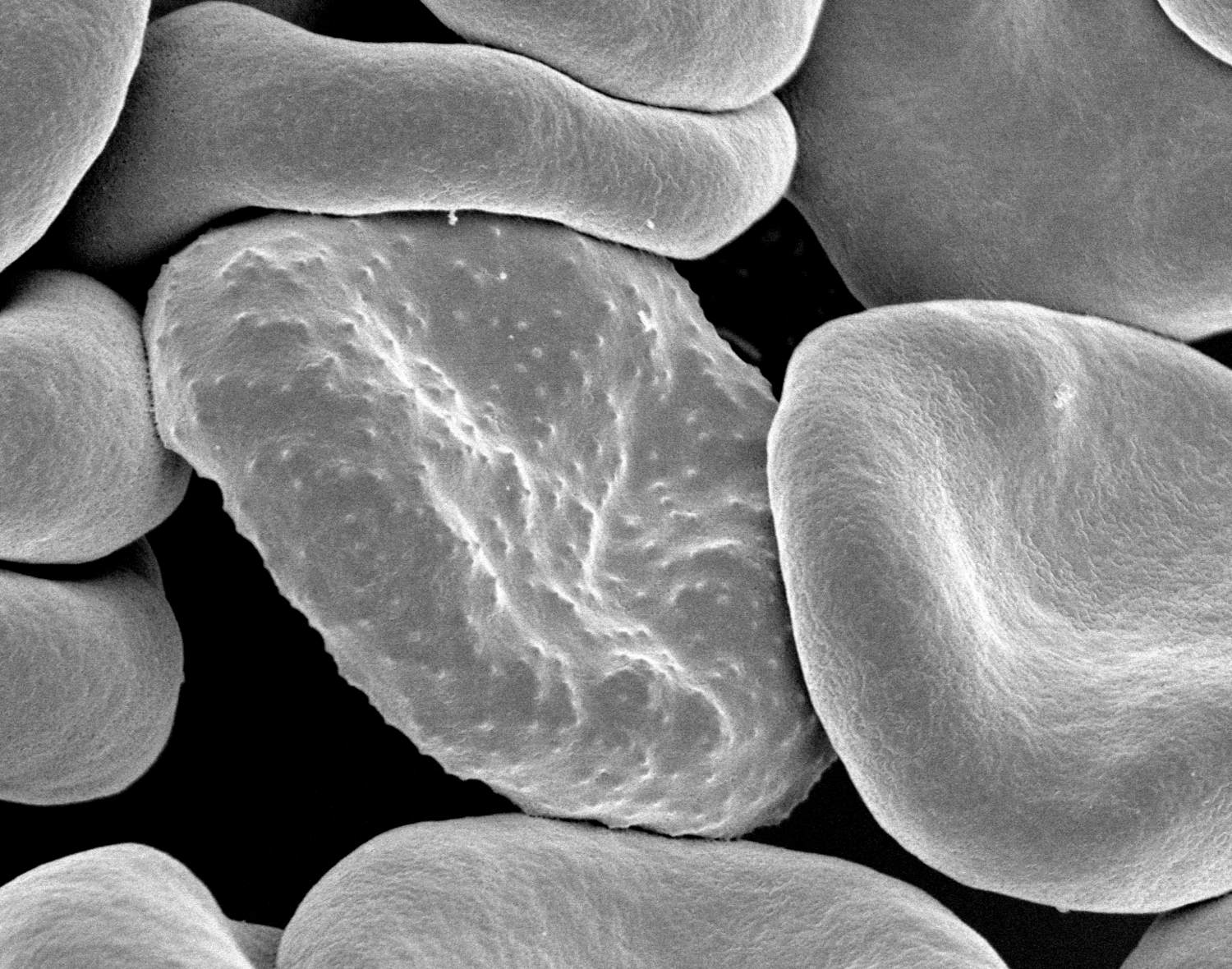
In June 2017, Betty Balikagala traveled to a hospital in Gulu District, in northern Uganda. It was the rainy season: a peak time for malaria transmission. Balikagala, a researcher at Juntendo University in Japan, was back in her home country to hunt for mutations in the parasite that causes the disease.
For about four weeks, Balikagala and her colleagues collected blood from infected patients as they were treated with a powerful cocktail of antimalarial drugs. After initial analysis, the team then shipped their samples — glass slides smeared with blood, and filter papers with blood spots — back to Japan.
In their lab at Juntendo University, they looked for traces of malaria in the blood slides, which they had prepared by drawing blood from patients every few hours. In previous years, Balikagala and her colleagues had observed the drugs efficiently clearing the infection. This time, though, the parasite lingered in some patients. “We were very surprised when we first did the parasite reading for 2017, and we noticed that there were some patients who had delayed clearance,” recalled Balikagala. “For me, it was a shock.”
Malaria kills more than half a million people per year, most of them small children. Still, between 2000 and 2020, according to the World Health Organization, interventions prevented around 10.6 million malaria deaths, mostly in Africa. Bed nets and insecticides were responsible for most of the progress. But a fairly large number of lives were also saved by a new kind of antimalarial treatment: artemisinin-based combination therapies, or ACTs, that replaced older drugs like chloroquine.
Used as a first-line treatment, ACTs have averted a significant number of malaria deaths since their introduction in the early 2000s. ACTs pair a derivative of the drug artemisinin with one of five partner drugs or drug combinations. Delivered together, the fast-acting artemisinin component wipes out most of the parasites within a few days, and the longer-acting partner drug clears out the stragglers.
ACTs quickly became a mainstay in malaria treatment. But in 2009, researchers observed signs of resistance to artemisinin along the Thailand-Cambodia border. The artemisinin component failed to clear the parasite quickly, which meant that the partner drug had to pick up that load, creating favorable conditions for partner drug resistance, too. The Greater Mekong Subregion now experiences high rates of multi-drug resistance. Scientists have feared that the spread of such resistance to Africa, which accounts for more than 90 percent of global malaria cases, would be disastrous.
Now, in a pair of reports published last year, scientists have confirmed the emergence of artemisinin resistance in Africa. One study, published in April, reported that ACTs had failed to work quickly for more than 10 percent of participants at two sites in Rwanda. The prevalence of artemisinin resistance mutations was also higher than detected in previous reports.
In September, Balikagala’s team published their report from Uganda, which also identified mutations associated with artemisinin resistance. Alarmingly, the resistant malaria parasites had risen from 3.9 percent of cases in 2015 to nearly 20 percent in 2019. Genetic analysis shows that the resistance mutations in Rwanda and Uganda have emerged independently.
The latest malaria report from the WHO, published in December, also noted worrying signs of artemisinin resistance in the Horn of Africa, on the eastern side of the continent. No peer-reviewed studies confirming such resistance have been published yet.

So far, the ACTs still work. But in an experimental setting, as drug resistance sets in, it can lengthen treatment by three or four days. That may not sound like much, said Timothy Wells, chief scientific officer of the nonprofit Medicines for Malaria Venture. But “the more days of therapy you need,” he said, “then the more there is the risk that people don’t finish their course of therapy.” Dropping a treatment course midway exposes the parasites to the drug, but doesn’t clear all of them, potentially leaving behind survivors with a higher chance of being drug resistant. “That’s really bad news, because then that sets up a perfect storm for creating more resistance,” said Wells.
The reports from Uganda and Rwanda have yielded a grim consensus: “We are going to see more and more of such independent emergence,” said Pascal Ringwald, coordinator at the director’s office for the WHO Global Malaria Program. “This is exactly what we saw in the Greater Mekong.” Luckily, Wells says, switching to other ACTs helped to combat resistance when it was detected there, avoiding the need for prolonged treatment.
A new malaria vaccine, which recently received the go-ahead from the WHO, may eventually help reduce the number of infections, but its rollout won’t have any significant impact on drug resistance. As for new drugs, even the most promising candidate in the pipeline would take at least four years to become widely available.
That leaves public health workers in Africa with only one solid option: track and surveil resistance to artemisinin and its partner drugs. Effective surveillance systems, experts say, need to ramp up quickly and widely across the continent.
But most experts say that surveillance on the continent is patchy. Indeed, there is considerable uncertainty about how widespread antimalarial resistance already is in sub-Saharan Africa — and disagreement over how to interpret initial reports of emerging partner drug resistance in some countries.
“Our current systems are not as good as they should be,” said Philip Rosenthal, a malaria researcher at the University of California, San Francisco. The new reports of artemisinin resistance, he added, “can be seen as a wake-up call to improve surveillance.”
Malaria drugs have failed before. In the early 20th century, chloroquine helped beat back the pathogen worldwide. Then, about a decade after World War II, resistance to chloroquine surfaced along the Thailand-Cambodia border.
By the 1970s, chloroquine-resistant malaria had spread across India and into Africa, where it killed millions, many of them children. “In retrospect, we know that chloroquine was used for many years after there was a huge resistance problem,” said Rosenthal. “This probably led to millions of excess deaths that could have been avoided if we were using other drugs.”
The scurry to find new drugs yielded artemisinin. Used by Chinese herbalists some 2,000 years ago to treat malaria-like symptoms, artemisinin was rediscovered in the 1970s by biomedical researchers in China, and its use became widespread in the 2000s.
Haunted by the failure of chloroquine, though, researchers have remained on the lookout for signs that the malaria parasite is evolving to resist artemisinin or its partner drugs. The gold-standard method is a therapeutic efficacy study, which involves closely monitoring infected patients as they are treated with antimalarial drugs, to see how well the drugs perform and if there are any signs of resistance.
The WHO recommends conducting these studies at several sites in a country every two years. But “each country interprets that with their capability,” said Philippe Guérin, director of the WorldWide Antimalarial Resistance Network at the University of Oxford. Efficacy studies are slow, costly, and labor intensive. Also, “you don’t get a very good geographical representation,” said Guérin, because you can do a new clinical trial in only so many places at a time.
To get around the problems associated with efficacy studies, researchers also turn to molecular surveillance. Researchers draw a few drops of blood from an infected individual onto a filter paper, then scan it in the laboratory for certain genetic mutations associated with resistance. The technique is relatively easy and cheap.
With these kinds of surveillance data, policymakers can choose which drugs to use in a particular region. Moreover, early detection of resistance can prompt health authorities to take actions to limit the spread of resistance, including more aggressive screening and treatment campaigns, and expanded efforts to control the mosquitos that spread malaria.
In practice, though, this warning system is frayed. “There is really no organized surveillance system for the continent,” said Rosenthal. “Surveillance is haphazard.”
In countries lacking a robust health care system or mired in political instability, experts say, resistance could be spreading undetected. For example, the border of South Sudan is just 60 miles from the site in northern Uganda where Balikagala and her colleagues confirmed resistance to artemisinin. “Because of the security issues and the refugee-weakened system, there is no surveillance that tells us what is happening in South Sudan,” said Guérin. The same applies in some parts of the nearby Democratic Republic of the Congo, he added.
In the past, regional antimalarial networks, like the now defunct East African Network for Monitoring of Antimalarial Treatment, have addressed some surveillance gaps. These networks can help standardize protocols and coordinate surveillance efforts. But such networks have suffered from recent lapses in donor funding. The East African network “will be awakened,” Balikagala predicted, as concerns about artemisinin-resistant malaria grow.
In southern Africa, eight countries have come together to form the Elimination Eight Initiative, a coalition to facilitate malaria elimination efforts across national borders, which may help jumpstart surveillance efforts there.
Ringwald said drug resistance is a priority for him and his WHO colleagues. At a malaria policy advisory committee meeting last fall, he said, the issue was “high on the agenda.” However, when pressed for answers on how the WHO plans to combat drug resistance in Africa, Ringwald emailed Undark an excerpt from the organization’s 2021 World Malaria Report. The report states that the WHO will “work with countries to develop a regional plan for a coordinated response,” but does not lay out any specifics on that response plan. The Africa Centers for Disease Control and Prevention, part of the African Union, did not respond to requests for comment on its plans to bolster surveillance.
“There is an ethical obligation to researchers, and to people responsible for surveillance, that if you pick up these problems, share them as quickly as possible, react to them as strongly as possible,” said Karen Barnes, a clinical pharmacologist at the University of Cape Town who also co-chairs the South African Malaria Elimination Committee. “And try very, very hard” to make sure “that it’s not going to be the same as when we had chloroquine resistance in Africa.”
In the absence of more robust surveillance, reports have also identified worrying — but, some scientists say, inconclusive — signs of partner drug resistance.
A series of four studies conducted between 2013 and 2019 at several sites in Angola found the efficacy of artemether-lumefantrine — the most widely used ACT in Africa — had dropped below 90 percent, the WHO threshold for acceptable malaria treatment. Peer-reviewed studies from Burkina Faso and the Democratic Republic of the Congo have reported similar results.
The studies have not found genes associated with artemisinin resistance, suggesting that the partner drug, lumefantrine, might be faltering. But several malaria researchers told Undark they were skeptical of the studies’ methods and viewed the results as preliminary. “I would have preferred that we look at data with a standardized protocol and exclude any confounding factors like poor microscopy or analytical method,” said Ringwald.
Mateusz Plucinski, an epidemiologist at the U.S. Centers for Disease Control and Prevention’s Malaria Branch who participated in the Angola research, defended the findings. “The persistence of artemether-lumefantrine efficacy near or under 90 percent in Angola likely suggests that there is likely a true signal of decreased susceptibility of parasites to this drug,” he wrote in an email to Undark. In response to the data, Angolan health officials have begun using a different ACT.
For now, it’s unclear how bad the situation is in Africa — or what the years ahead could bring. The research community and the authorities are “at the level of just watching and seeing what happens at this stage,” said Leann Tilley, a biochemist at the University of Melbourne who researches antimalarial resistance. But experts say that if artemisinin resistance does flare up and starts impinging on the partner drug, policymakers might need to consider changing to a different ACT, or even deploy triple ACTs, with two partner drugs.
Some experts are hopeful that artemisinin resistance will spread more slowly in Africa than it has in southeast Asia. But if high-grade resistance to artemisinin and partner drugs were to arise, it would put Africa in a bind. There are no immediate replacements for ACTs at the moment. The Medicines for Malaria Venture drug pipeline has about 30 molecules that show promise in preliminary testing, and about 15 molecules that are undergoing clinical trials for efficacy and safety, said Wells. But even the drugs that are at the end of the pipeline will take about five to six years from approval by regulatory authorities to be incorporated into WHO guidelines, he noted — if they make it through trials at all.
Wells cited one promising compound, from the drugmaker Novartis, that recently performed well in early clinical trials. Still, Wells said, the drug won’t be ready be deployed in Africa until around 2026.
Funds for malaria control and elimination programs remain limited, and scientists worry that, between Covid-19 and the malaria vaccine rollout, attention and resources for conducting surveillance and drug resistance work might dry up. “I really hope that those that do have resources available will understand that investing in Africa’s response to artemisinin resistance today, preferably yesterday, is probably one of the best places that they can put their money,” said Barnes.
The annals of malaria have shown time and again that once resistance emerges, it spreads widely and imperils progress against the deadly disease. For Africa, the writing is on the wall, said Barnes. The bigger question, she asked, is this: “Are we capable of learning from history?”
Pratik Pawar is an independent science journalist based in India. His work has been published in Science News, Discover, The Wire, and The Washington Post, among others.











Comments are automatically closed one year after article publication. Archived comments are below.
Dear Pratik Pawar,
It is upsetting to read that malaria still kills more than half a million people a year. Another sad fact is that 85% of all people dying from malaria are among the poorest 20% of the population.
You mention artemisinin, the active ingredient of the herb Artemisia annua. Artemisinin is one of many constituents in this medicinal herb. The whole herb Artemisia annua as a herbal infusion has a very high success rate in treating malaria (about 97%). Have you not come across it in your research? Or do you think it is not worth mentioning this valuable and effective herb? The WHO claims that the use of the herb Artemisia annua leads to rendering the pharmaceuticals less effective and increases the problem of resistance. During hundreds of years the use of the whole herb has NEVER lead to resistance. Why do you not advocate the use of this herbal tea? Why do you not even mention it? You talk of excess deaths. Why not offer this tea which is cheap and effective and why not encourage people in malaria-prone areas to grow this herb? The indigenous African version of Artemisia is Artemisia afra which has been used in the past to treat malaria. Artemisia afra is a perennial herb, grows in Africa and does NOT contain Artemisinin and is still effective as a herbal tea to treat malaria. If every family was able and encouraged to grow Artemisia annua or Artemisia afra the number of excess or unnecessary deaths would most likely decrease.
Yours sincerely, Luzia