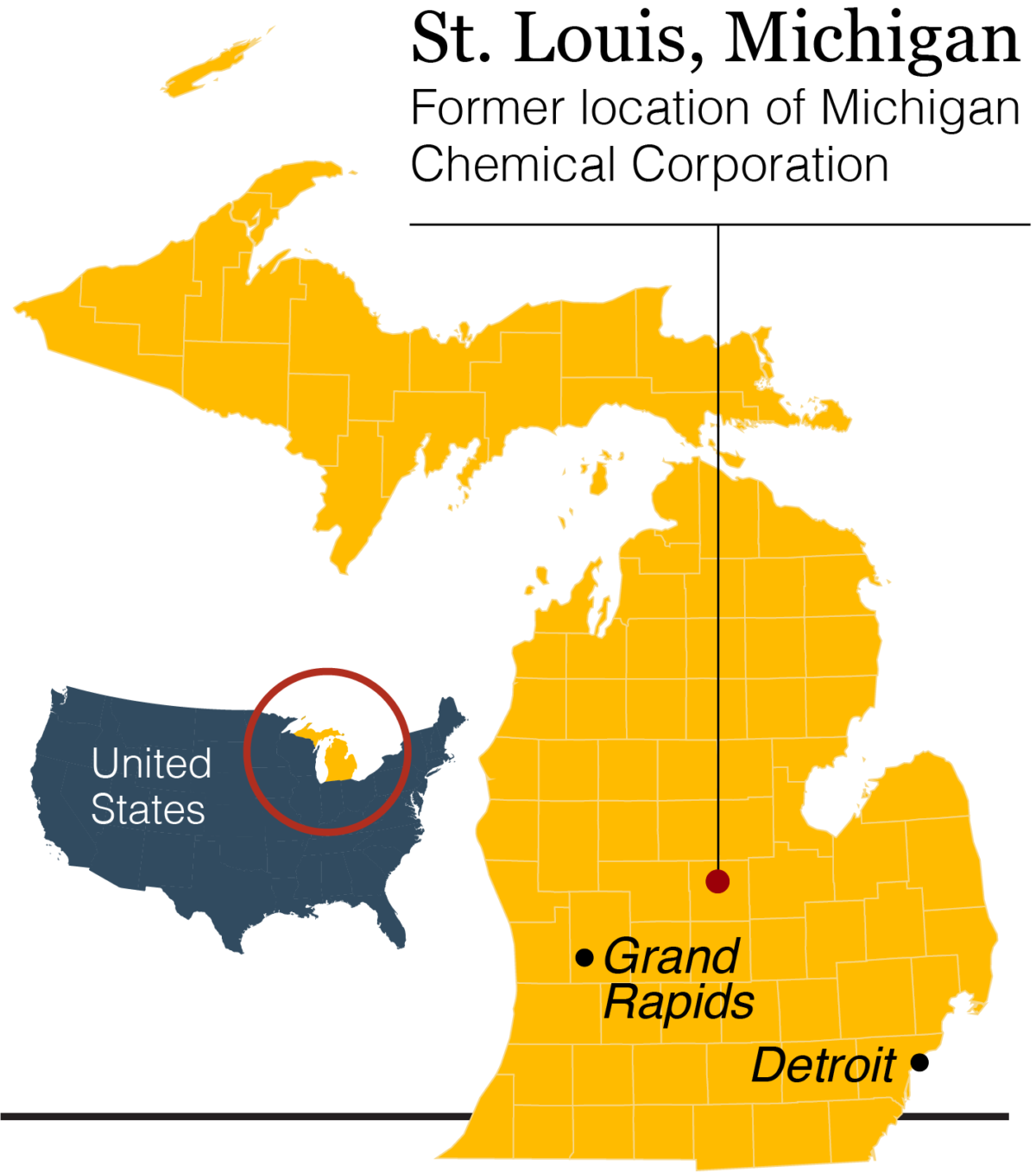
Uncertain Inheritance: Epigenetics and the Poisoning of Michigan
Jim and Ida Hall buried their daughter Jerra in a family plot at the bottom of a grassy rise. Several times a year, Jim Hall drives just over a mile from his home on North Main Street in the town of St. Louis, Michigan to Jerra’s headstone in the back corner of Oak Grove Cemetery in his 1997 Chevy pickup. In the 12 years since complications from a rare heart defect claimed the life of their brown-haired toddler, her family continues to cover her grave with stuffed animals (frogs were her favorite). Hall gently sweeps off the leaves and debris covering the childhood paraphernalia and wipes his callused hands on a pair of worn jeans, his tall frame stooped by grief. He stops and stares at the inscription: “Two years, two months, too little.”
“We didn’t know what else to write,” he said.

Jim Hall’s exposure to PBB as a child makes him valuable in the hunt for the answer to a burning scientific question: Can a father’s exposure to environmental toxins impact the health of his progeny?
Visual: Jeffrey Sauger for Undark
Jerra’s headstone sits where an umbrella of majestic oaks gives way to the dreadlocks of vines and grasses of a small wetland in the geographic center of Michigan’s Lower Peninsula, a little more than a mile from the chemical plant that once produced a toxic flame retardant called PBB, short for polybrominated biphenyl. Hall can’t help but think it may have killed his little girl.
“When your daughter is born with a heart condition and doesn’t survive,” he said, “you just wonder.”
The problem: Baby Jerra herself was never exposed to PBB. In fact, she was born some 30 years after the Michigan Chemical Corporation, headquartered in St. Louis, accidentally mixed several hundred pounds of PBB into livestock feed and subsequently stopped producing the chemical. Until a few years ago, a granite tombstone marked the shuttered site.
Still, in the early 1970s, virtually all of the Lower Peninsula’s 8.5 million residents consumed meat, milk, and eggs contaminated with PBB before the mistake was discovered. The Michigan Department of Agriculture called it “the most costly and disastrous accidental contamination ever to occur in United States agriculture.” Hall, who had just turned 11 in 1974 when the mix-up was uncovered, was among those residents — and because he lived just a few blocks from the plant, he likely got an extra dose. His grandparents’ home, where he often stayed, was even closer: just three houses away and directly downwind.
The exposure almost certainly caused chemical changes to his genes, which affected not the sequence of letters in his genome, but rather how those genes are switched on and off through epigenetic information that sits on top of DNA. Recent animal studies have suggested that epigenetic changes could be passed down through generations, potentially causing inherited health problems in children, grandchildren, and great-grandchildren.
And yet, the science behind transgenerational epigenetic inheritance, as such a transfer of maladies would be known, remains highly controversial, and researchers have never been able to prove its existence in humans. That’s not for want of trying, however, and Michele Marcus, an epidemiologist at Emory University, believes the PBB mix-up in Michigan could prove crucial to that effort. Her preliminary work investigating this macabre, natural experiment has already suggested that the children of exposed mothers have many health problems possibly linked to PBB exposure. But the real question mark remains men like Hall, who were exposed to the chemical as children, grew up and married women who weren’t, and then had children themselves.
Could those offspring — who were never exposed to PBB in the 1970s, and who were never exposed to even residual PBB while developing in the wombs of exposed mothers — nonetheless experience health impacts linked to the chemical? It’s a question with profound implications.
“If you look at it through the lens of environmental justice, it changes the debate,” Marcus said, “because it’s not just, ‘Okay. I can make a decision to work in an industry that might be hazardous to my health,’ but ‘Am I making that choice for my grandchildren?’” There are people who don’t have a choice about being exposed, she added.
At the time of the incident, little was known about the biological effects of PBB exposure, and even today, only a handful of the 84,000 chemicals currently approved for use in manufacturing and industry have ever undergone real regulatory scrutiny. Even in the small subset of chemicals that have, government toxicologists mainly check for acute health effects on human populations, and while there are some testing procedures for chronic exposures, the potential impacts three generations on is not even a consideration. Now, with President Donald Trump’s proposed budget cuts at the Environmental Protection Agency, the chemical industry will likely enjoy only more freedom, and less scrutiny — a prospect that a growing number of scientists worry could reverberate across generations in ways that we are only at the earliest stages of understanding.
Visual: Undark
For researchers like Marcus, this makes grappling with the potential echoes of Michigan’s PBB exposure all the more urgent — and not least because kindred chemicals remain in wide circulation. Although PBB itself fell out of use in the years following the Michigan incident, other brominated flame retardants, including polybrominated diphenyl ether (PBDE), are ubiquitous in everything from electronics and vehicles to furniture and textiles. Both PBB and PBDE are persistent organic pollutants, a large class of compounds that includes chemicals like DDT, polychlorinated biphenyls (PCBs), and dioxins that resist being broken down in the body and the environment. And if Marcus finds that PBB can affect multiple generations — even those with no direct exposure to the chemical — “it’s probably going to be true for a lot of other chemicals,” she said.
If Marcus is right, it could upend not just medicine, but whole strata of legal, regulatory, and even ethical bedrock. Could insurers refuse coverage to the great-grandchildren of people exposed to a chemical toxin? Where would liabilities end? What are the real-world implications of personal health problems linked not to some chemical exposure that unfolded in our lifetimes, but at some distant point in our past lineage? “It changes the rules,” said Joe Nadeau, a geneticist at the Pacific Northwest Research Institute, a nonprofit biomedical research facility based in Seattle. “It’s one thing to have the direct exposure. We all know that, we all worry about it,” Nadeau said. But if the consequences of those environmental exposures can be delivered to our children or grandchildren, then we don’t just have ourselves to consider. “We have a responsibility to do better,” he said.
Hall feels that responsibility more deeply than most. When his daughter was sick, doctors told him that her heart defect was just dumb luck, and Hall had no reason to search for alternate explanations. But when he joined a community group working to clean up the contaminated Michigan Chemical site, Hall began to connect the dots between Jerra’s illness and all the PBB he had inhaled and ingested as a child.
“I hope that we can get research on [those of] us that have been affected,” he said, “so that this can be figured out.”

That the legacy of PBB exposure hangs like a dark cloud over generations of families — and over vast areas of rural Michigan — is a profound understatement. The stories vary in their details, but most begin with a slow awakening to the idea that something wasn’t right. Maybe the cows were acting funny, or the chickens seemed off. And then, as livestock began to die, the awakening tended to grow into a certainty that was typically met, at least at the outset, with patronizing dismissal by authorities.
That’s how it went down for dairy farmer Frederic Halbert. Dawn broke with hesitation on Thursday September 20, 1973 through overcast skies as Halbert traveled the five miles from the family’s farmhouse to the milking parlor. As the first group of black-and-white cows ambled in, they seemed lethargic, and they didn’t walk so much as stagger unevenly across the parlor.
Despite weighing in at 1,500 pounds, Holsteins have delicate stomachs. Since the quality of a cow’s diet directly impacts the quality and quantity of milk it produces, Halbert paid close attention to what his cows ate. It’s why he ordered 65 tons of a new cattle feed — called Dairy Ration 402 — from the Michigan Farm Bureau between the end of August and the first week of October 1973. Halbert, who had worked as a chemical engineer before returning to dairy farming, had grown interested in the new cow chow — a mix of grains and vitamins — because it contained small, silvery pellets of magnesium oxide to improve digestion and boost milk production. Halbert used DR402 in the fall and winter to supplement the farm’s home-grown fodder. Not long after, the cows stopped eating and grew listless in the spacious, open-air barn. Milk production plummeted. Halbert’s vet ran countless tests and submitted samples and entire animals for analysis by the state, none of which provided any concrete answers.
“My dad’s herd was his life,” said Lisa Halbert, Frederic’s daughter, who was only a toddler at the time. “He knew something was wrong, and he felt that no one was giving him any answers.” (Both journalist Joyce Egginton and Halbert himself would chronicle his long search for answers in books, “The Poisoning of Michigan” and “Bitter Harvest.”)
Across the state, dairy farmers were noticing similar behaviors in their herds. Cows began falling ill. But with no diagnosis — no infectious disease or concrete factor to blame — veterinarians and the Farm Bureau suggested the illnesses may be caused by fungus-contaminated feed from a cold, wet summer, or by bad animal husbandry. Halbert knew that wasn’t the case, and after months of testing turned up nothing, he turned to DR402 as the culprit, since it was the only recent change to his cow’s diets.
He sent samples to labs around the country, including to the U.S. Department of Agriculture’s National Animal Disease Center in Ames, Iowa. In January 1974, their tests revealed an unknown chemical contaminant: an unusually heavy compound that no one could discern. Analysis from the Wisconsin Alumni Research Foundation (WARF) also showed that DR402 from Halbert’s farm contained very little of the magnesium oxide that had been listed among the feed’s ingredients. When the Ames lab ran out of funding to identify the contaminant in DR402, feed was also sent to USDA scientist George Fries in Maryland, who identified the mystery chemical as a type of polybrominated biphenyl (PBB). Halbert immediately realized what had happened, since Michigan Chemical made both magnesium oxide and PBB.
Instead of shipping magnesium oxide to the Farm Bureau, Michigan Chemical had accidentally shipped several thousand pounds of a flame retardant marketed under the brand name Firemaster. To the naked eye, the Firemaster pellets looked for all the world like harmless magnesium oxide mixed in with the DR402 feed — but it wasn’t harmless.



PBBs are dumbbell-shaped compounds consisting of two attached carbon rings (the phenyl groups) with varying numbers of bromine atoms linked to either end. When added to children’s clothing, upholstery fabric, or electronics, they interfere with the item’s ability to catch fire, making them one of the first commercial flame retardants and providing Michigan Chemical with a veritable goldmine. Still, an internal memo revealed that corporate chemists had expressed safety concerns if PBBs were ingested or inhaled. In 1968, people in Japan ate rice bran oil contaminated with chemically similar compounds including polychlorinated biphenyls (PCBs). After 14,000 people in Fukuoka Prefecture reported difficulty breathing, skin rash, weakness, and skin discoloration, public health officials discovered PCBs flowing through coils used to heat the oil.
PBBs, PCBs, and related chemicals are stored in the body fat and not excreted out, which gives them decades to cause health effects. Because PBBs were never meant for the food supply of humans or animals, however — and given the industrial bravado of the 1970s — Firemaster was marketed as safe. When Michigan Chemical learned that the PBB issue had been traced back to their plant, they disposed of PBBs in the county landfill and in a nearby burn pit that now sits on a golf course. Both are now designated as Superfund sites. The company stopped producing PBBs for good on November 20, 1974.
Six months before that, the Michigan Department of Agriculture began quarantining dairy farms whose milk products were found to have PBB levels greater than 1 part per million. By November, that level had been lowered to .3 parts per million. Although the state did not mandate that farmers have their quarantined animals killed (supposedly to prevent them from taking legal action), farmers felt they had little option but to allow the state to collect their animals, truck them to the northern tip of the Lower Peninsula in Kalkaska, shoot them, and bury them in mass graves.
Other livestock, including chicken (and their eggs) and pigs, were implicated in the feed contamination, multiplying the exposures — and the animal executions — across the state. The exposures included not just farm families, but consumers who ate contaminated beef or chicken or pork, or who consumed contaminated milk, cheese, or eggs, as well as workers who produced the chemical in factories — and their spouses and offspring.
Direct exposure to PBBs, whether through consumption or absorbed through skin or inhalation, has been linked to an increased risk for a variety of ailments, from short-term incidence of skin rashes, hair loss, and muscle and joint issues, to longer term thyroid problems and changes in hormone levels.
Lisa Halbert is circumspect about her own health issues as an adult and the PBBs that her father unwittingly imported to their dairy cow operation when she was a child. She’s blonde and broad-shouldered, and she greeted me in knee-high rubber boots and a forest green Lorax shirt that she wore to the March for Science in Washington earlier this year. Now in her mid-40s, Halbert had taken a medical leave from her work as a dairy veterinarian to recover from hip replacement surgery and a hysterectomy.
“I got spayed,” she quipped.
Her memories of the PBB disaster remain vivid. When she learned as a child that her favorite cow, named Flopsy, was to be included in the state’s quarantine-and-slaughter action, she says she and her sisters discussed ways to save her, including sneaking her into the woods and then borrowing a truck to move her somewhere safe. But the oldest Halbert sister was a mere seven years old, and Flopsy was quickly collected and dispatched to Kalkaska.
A documentary film shot shortly after the PBB contamination was discovered captured the gruesome process by which thousands of cattle had to be destroyed.
The family eventually removed the feeding station where their doomed cows ate the contaminated DR402. Today the parlor still stands, but is not used to collect any milk sold for human consumption. Lisa Halbert recalls the family bulldozed one of the barns used to house cows during the quarantine phase, but many of the other outbuildings still stand.
The elder Halbert, meanwhile, is now a terse, moon-faced man in his 70s. He lives about nine miles from the dairy, situated just north of Battle Creek, and a tour of the grounds today reveals no signs, overt or covert, of the contamination that forever altered the family.
By comparison to these sorts of experiences, Jim Hall and his family were far removed from the PBB drama as it was initially unfolding. And because he didn’t live on a contaminated farm or work in the factory, epidemiologists at the Michigan Department of Community Health, which established the Michigan PBB registry in 1976 to gather and analyze data on exposed residents, didn’t recruit Hall to be part of their study cohort.
Still, Hall’s brother developed Hodgkin’s lymphoma in the early 1980s, not far from a cluster of cancer cases around the town of Breckenridge, five miles east of St. Louis. In 2008, five years after the birth of his daughter — and three years after the family buried her — Hall found a lump in his throat while driving. When he went to the doctor complaining of fatigue and difficulty swallowing, he found out that precancerous nodules adorned his thyroid gland like ornaments on a Christmas tree. Doctors removed it.
Initial data gathered from the PBB cohort study revealed other health problems among the hundreds of families and thousands of individuals recruited. Like other infamous toxicants such as bisphenol A and dioxin, PBB is classified as an endocrine disruptor, meaning that it interferes with the body’s array of natural hormones. Growing concern about endocrine disruptors since the 1990s has spurred an avalanche of research linking these chemicals to thyroid problems, diabetes, obesity, fertility problems, changes in pubertal development, and hormone-sensitive cancers such as breast and prostate cancer.
“We can detect a very large number of chemicals in essentially everybody’s bodies,” said Jonathan Chevrier, an environmental health scientist at McGill University. “Chemicals that we have reason to believe may interfere with hormones, for instance. That’s complete news to most people.”
Studies launched from the Michigan Department of Community Health’s long-term monitoring of affected residents revealed a variety of findings. Some research has shown an elevated prevalence of liver, neurological, and immunological problems among Michigan farm families, but these issues have not been consistently linked to PBB blood levels. A 2011 study by Marcus and others at Emory University found that women exposed to PBB in utero were more likely to experience miscarriages. A few other early studies — all on small groups of children — suggested that those exposed to PBBs were more likely to have developmental delays. But for all this work, in 2011, the study hit a crisis point. The state had run out of funding and, with the disaster increasingly confined to the dusty archives of history, it seemed like little more could be learned.
Michele Marcus, though, disagreed. Trained as a reproductive and environmental epidemiologist, Marcus had worked alongside Irving Selikoff at the Mount Sinai School of Medicine in New York, years after he conducted some of the first investigations into the human health effects of PBB. As a young professor at Emory University working with the CDC in the mid-1990s, she began investigating how PBB exposure continued to affect health after 20 years. As someone interested in how early life experiences shaped a person’s long-term health, Marcus knew that the decades-long study provided an invaluable set of data on the long-term health impacts of a chemical exposure. She eventually took over the research and the Michigan PBB Registry now resides at the Rollins School of Public Health at Emory University.

“Because I knew about the PBB incident, I thought: Wow, these people were exposed to a chemical we think is an endocrine disruptor. Maybe they might be experiencing these problems, and this would be a much stronger type of evidence, because we could measure individual exposure levels and look at the relationship to individual health outcomes. It’s much stronger evidence than just looking at trends over time, because over time, lots of things are changing,” Marcus said.
Given her training, Marcus was also well aware of the sensitivity of developing organisms. In 1995, she proposed to study the health issues affecting daughters of women who had been exposed to PBB. But it wasn’t until 2008 that her participation in a meeting in Washington, D.C. for the Institute of Medicine (now the National Academy of Medicine) introduced her to the idea that a father’s exposure to PBB could have an effect on his children. No one had verified the idea in humans, but Marcus’s PBB cohort, she was convinced, provided the perfect case study.
To understand why, it helps to cast back nine years before that 2008 meeting, to the spring of 1999. It was then that Michael Skinner, a reproductive biologist at Washington State University in Pullman, recalls one of his postdocs, Andrea Cupp, bursting through his office door. Skinner and Cupp had been working to understand the process of how a fetus became male or female, and injected pregnant rats with a variety of hormone mimics, including methoxychlor (a pesticide) and vinclozolin (a fungicide), to study how the process was disrupted. The resulting adult males had low sperm counts and reduced fertility, but these were not the type of groundbreaking results either scientist was looking for. Cupp’s next task was to breed the males exposed to vinclozolin in the womb (the F0 generation) to check for effects in their offspring — rats that hadn’t been directly exposed to the chemical.
As it would turn out, however, Cupp had accidentally bred the children of the F1 rats by mistake, creating what would be considered an F2 generation. Visibly upset, she had come to Skinner’s office to explain the mix-up. He told her to analyze the rats anyway, figuring that the task would help take her mind off the error. Several weeks later, Cupp hustled into Skinner’s lab again, this time brimming with excitement. The testes on the F2 rats, she had discovered, looked exactly like those on the F1.
“Of course, I didn’t believe her and I made her go back and repeat it 15 times,” Skinner now recalls. But no matter how many times Cupp bred the rats, out to F4, 90 percent of the male offspring had reduced sperm counts and lowered sperm motility — even though they themselves had never been exposed to vinclozolin. “Then I knew that we had stumbled on something that was important,” he said.
Cupp and Skinner now had to figure out how this was happening. The high rate of testicular abnormalities ruled out direct DNA mutations, which were more random and happened at a lower frequency. That left the duo with a type of genetic change that decades of scientific dogma told them wasn’t possible. British biologist Conrad Waddington first coined the term epigenetics after performing an experiment involving fruit flies and heat. A horizontal vein bisects the miniscule wings on the poppy seed-sized insect, controlled by a gene named crossveinless. Waddington made this vein disappear in newly bred generations of fruit flies by repeatedly heating the pupae. In breeding those flies that lacked the bisecting vein, he discovered that their offspring had the same trait, despite never being exposed to heat themselves. His analysis didn’t reveal any mutations in the genetic sequence itself, so he called it an “epigenetic” phenomenon — a way of describing genetic changes occurring above and beyond the ordering of genes.
After the discovery of DNA’s double helix, biologists had already shown that small chemical tags called methyl groups could act as epigenetic markers on the genome — punctuation marks, in a sense, on the cascading paragraphs of A-C-G-T that make up our genetic code. Another analogy used by researchers at Harvard Medical School: “If DNA is like a book, epigenetic marks are like sticky notes. Epigenetic marks tell our cells whether and how to read the genes.”
Get Undark's weekly newsletter, delivered right to your inbox!
Carrie Breton, an epigeneticist and environmental health scientist at the University of Southern California, adds the key insight that folks like Skinner and Cupp and Waddington were uncovering: “These marks are fluid, not static,” she told me. “It’s yet another layer of complexity on top of the genetic code.”
That complexity came as something of a surprise. Work by developmental biologists in the 1980s had long suggested that newly fertilized embryos appeared to strip all the methyl groups off their genomes, essentially creating a blank slate from which to work. By this understanding, the multigenerational epigenetic inheritance observed by Cupp and Skinner was technically impossible. In the intervening quarter century, however, it had become more and more evident that an embryo’s epigenetic slate wasn’t nearly as blank as researchers thought.
Angelman syndrome, characterized by a small head, seizures, and developmental disabilities, for example, is caused by a deletion in chromosome 15 in the mother. The exact same mutation inherited from the father causes Prader-Willi syndrome, which causes an insatiable appetite. (Both genetic disorders can also be caused if a child inherits two copies of a section of the chromosome from the father and mother, respectively, rather than one from each.) Some type of marker had to exist that distinguished the parent from which the mutation arose. To date, scientists have discovered more than 100 different imprinted conditions in humans. This type of genomic imprinting provided the first indication that epigenetic markers could be inherited.
Skinner’s study provided more conclusive evidence, and when he and Cupp finally published their work in Science in 2005, the study made an immediate splash. Skinner’s phone rang off the hook with calls from reporters from around the world. Six months later, scientific and industry pushback began. Critics pointed out that Skinner injected the animals with vinclozolin rather than lacing their food. Since humans were exposed to the fungicide by ingestion or inhalation, not injection, the harmful effects to the rats may not mimic human issues. A postdoc found guilty of scientific misconduct in 2010 only fueled the fire.
Still, when other labs conducted related experiments with different chemicals, they found broadly similar results. “I think it sank in that what I was telling them was going to affect lots of different things,” Skinner said.
“This changes how you think about toxicology,” he later added. “The whole system changes.”

Skinner began testing other environmental compounds, including DDT, bisphenol A, and dioxin (including Agent Orange), for their ability to induce multigenerational epigenetic changes in rats. In almost every case, he found them. Importantly, each chemical created its own unique epigenetic fingerprint on the rat’s DNA, giving scientists the opportunity to potentially investigate what specific compound an individual was dosed with.
His work in this area placed him in high demand at universities and scientific conferences. In 2008, he spoke at the Institute of Medicine meeting in Washington, D.C., where Michele Marcus first learned of his work. As part of a committee looking into the health effects of Agent Orange exposure among Vietnam veterans, Marcus attended as an expert in the long-term health effects of early life environmental exposures, and as Skinner talked about his research, she immediately began thinking about the PBBs in Michigan.
By that time, Marcus had already tracked a series of health issues in the children of women exposed to PBB. In one study, she and her co-authors found that daughters who were breastfed and exposed to high levels of PBB in the womb started their first periods an average of one year earlier than their counterparts who were exposed to lower levels. They were much more likely to have difficulties carrying a pregnancy to term. Sons were more likely to have urogenital birth defects. Both genders showed a high prevalence of thyroid problems associated with PBB exposure. Overall, six in ten Michiganders in the 2000s had PBB blood levels that were higher than 95 percent of the non-exposed population.
But poor health outcomes in the offspring of Michiganders first exposed to PBBs — if those outcomes were related to the initial contamination at all — would have been the result of direct exposures, too. To show multigenerational effects, Marcus would need to find grandchildren of those exposed to PBB in the 1970s.
As soon as Marcus heard Skinner’s talk on dioxin epigenetics in rats, however, she began to wonder whether epigenetics could also be playing a role in causing poor health outcomes down the generational lines of these Michigan families. Could the children and grandchildren and great-grandchildren of these exposed populations manifest with PBB-related health issues even though they themselves had never come in contact with the chemical?
Marcus teamed up with Alicia Smith, a geneticist at Emory University, to begin investigating whether PBB affected an individual’s epigenetic regulation, and whether those changes could be delivered across generations. After repeatedly being asked in community meetings about the effects a father’s exposure could have on his offspring, she and Smith secured a grant from the NIH and headed up to Michigan to start collecting fresh blood samples.
Marcus wasn’t the only one paying close attention to Skinner’s work in those years. Hearing him speak at an earlier National Academy of Sciences workshop, bioethicists Mark Rothstein of the University of Louisville and Gary Marchant of Arizona State University both say they immediately grasped the immense implications of this line of research. Rothstein recalled asking an industry representative in attendance about the potential legal impacts of epigenetic inheritance, and whether chemical manufacturers could one day be held liable for impacts on individuals removed from exposures not just by years, but by whole generations.
“All the blood seemed to drain from his face when I asked that question,” Rothstein said, “because now I’m suggesting that there’s kind of unlimited liability for manufacturers to as yet unborn generations.”

A 2009 paper co-authored by bioethicists Mark Rothstein and Gary Marchant raised numerous pressing questions with regard to transgenerational epigenetic transfer. But the question that loomed over everything was how this issue would be handled by the courts.
After the meeting, Marchant and Rothstein delved through the scientific literature to find other writing on the bioethics of epigenetics. When they couldn’t find anything, they decided to write one of their own. Their resulting paper, co-authored with Marchant’s student Yu Cai and published in Health Matrix: Journal of Law-Medicine in the winter of 2009, proposed more questions than it answered. Were epigenetic test results protected under the Genetic Information Nondiscrimination Act? (Probably not). How did multigenerational epigenetic inheritance affect the environmental justice movement? How could scientists use these findings without stepping over the line into eugenics?
The question that loomed over everything, however, was how this issue would be handled by the courts. To try and answer that issue, legal experts have often turned to another toxic chemical that seemed to produce multigenerational effects that, while not epigenetic in nature, might provide a model for tracing liability in epigenetic cases.
In 1966, pathologist Robert Scully asked 35-year-old Arthur Herbst, then a gynecological oncologist at Massachusetts General Hospital, about some bizarre tumors he had seen. A handful of girls, ranging in age from 15 to 22, had been diagnosed with a rare type of vaginal cancer called clear-cell adenocarcinoma that had, until this point, only been identified in post-menopausal women at MGH. Scully, Herbst, and some of their colleagues assembled these cases into a formal study to figure out what was going on. The answer came not from some dusty medical journal but rather a question from one of the girls’ mothers. She asked Herbst if the cancer could be associated with the DES she took while pregnant.
DES is short for diethylstilbestrol, a synthetic form of estrogen first developed by British chemists in 1938. Three years later, the FDA approved it for the treatment of “estrogen-deficient conditions” in both humans and livestock. After a small animal study hinted that low estrogen might cause miscarriages, obstetricians began prescribing it to their female patients with high-risk pregnancies. Marketing campaigns by manufacturers like Wyeth and Eli Lilly and Company touted DES as a new wonder drug, and women with normal, healthy pregnancies soon began taking it. A 1953 study seemed to suggest that DES didn’t prevent miscarriage, but it seemed like such a benign drug that doctors continued to prescribe it and women continued taking it into the 1970s.
Many of the studies that look at DES ask daughters if their mother took the drug. “Half the time the mom didn’t even know what she was given,” said Linda Titus, a cancer researcher at Dartmouth College who is heading up much of the research. “That was back in an era, roughly 1940 to 1970, when women didn’t ask too many questions.”
When Herbst investigated the mother’s question, he found that a small group of daughters of women who took DES while pregnant were astronomically more likely to develop clear-cell adenocarcinoma. The resulting paper, published on April 22, 1971 in the New England Journal of Medicine with the bland title “Adenocarcinoma of the Vagina,” became a landmark in the scientific literature.
“This is the first time a drug given to the mother was associated with the subsequent development of the malignancy in her offspring, in humans,” said Herbst, now in his mid-80s, who continues his work on DES at the University of Chicago. “It’s clear that the fetus doesn’t react like a mature adult. The growing embryo and fetus are sensitive to things that sometimes we don’t know about.”
Further studies revealed an increased risk of urogenital abnormalities in both the sons and daughters of women who took DES, as well as an increased risk of breast cancer in the DES mothers. To win a legal judgement, however, the plaintiffs needed to prove that DES caused their health problems. Apart from clear-cell adenocarcinoma, many of the harms reported by DES mothers and their children have a range of causes.
Epidemiological studies, according to Titus, have been able to show a strong association between prenatal exposure to DES and certain negative health outcomes. This work allows researchers to infer causation, but it generally cannot prove the exact cause of a disease in a particular individual. Titus points out that because some DES-related conditions are so rare and so strongly associated with prenatal exposure to the drug, in some cases epidemiologists can be “reasonably confident” of the link. To hold up from a legal standpoint, the courts ruled that epidemiological studies must show that exposure to a toxin more than doubles the risk of the resulting health problem — and that’s no easy task.
There are few “relative risks higher than 1.5 in many disease areas,” Titus said.
DES mothers and children who wanted to sue the manufacturers for damages faced other legal hurdles. Many times, several decades had elapsed between when the mothers took DES and when they filed their claims. As well, many companies sold DES between 1941 and 1971, and women rarely knew who manufactured the specific pills they took. In the 1980 case Sindell v. Abbott Labs, the California courts settled this issue by holding DES manufacturers liable for their proportion of the market share of the drug. If Company A made 40 percent of the DES sold in a particular area, they would pay 40 percent of the judgement awarded.
The success of DES mothers and daughters in winning tens of millions of dollars in class-action lawsuits, along with an opening of investigations into the possible harm to DES grandchildren, spurred interest in lawsuits by the third generation. In 1991, lawyers for nine-year-old Karen Enright, who was born prematurely and subsequently developed cerebral palsy, brought a case against DES manufacturer Eli Lilly. The grandmother took DES in 1959 while she was pregnant with her daughter, born in 1960.
Karen herself was born in 1981, and the suit alleged that the daughter’s reproductive tract abnormalities caused the granddaughter’s condition. In a six-to-one ruling in 1991, the New York Court of Appeals rejected the suit on philosophical grounds. Writing the majority opinion, Chief Judge Sol Wachtler noted that, “For all we know, the rippling effects of DES exposure may extend for generations. It is our duty to confine liability within manageable limits. Limiting liability to those who ingested the drug or who were exposed to it in utero serves this purpose.”



The idea of proximate causation is implicit in the Enright ruling, according to Steve Gold, a professor of environmental law at of Rutgers Law School. Tort law was developed to address typically immediate harms from actions, dealing well with cases such as a broken arm from playground equipment or severe burns from hot coffee. Cause occurs immediately before effect. Many environmental harms, even those that affect those directly exposed, often take decades to show up. Injury to the children and grandchildren of these individuals can be too far removed for courts to agree that the manufacturer should be liable. Multigenerational suits “offend many people’s sense of justice. It’s valuable [to the courts] to make liability finite,” Gold said.
Third-generation DES lawsuits aren’t based on epigenetics, but the courts are likely to apply similar principles to plaintiffs in any multigenerational epigenetics cases. “There’s no fundamental legal issue that would interfere with a lawsuit,” Gold said. “[But] by the time you’re three generations on, finding proof that an exposure caused harm is going to be difficult to find or show.”
Still, Texas-based attorney Andrew Lipton points out that the revolution in the scientific, popular, and legal understanding of genetics could alter a judge’s willingness to hear a case based on epigenetic evidence. A solid case depends on demonstrating evidence of exposure both via historic records and via every chemical’s unique epigenetic fingerprint. Epidemiologists also need to provide proof that this fingerprint isn’t caused by other environmental exposures and that it leads to harm.
Lipton believes that lawsuits based on multi-generational epigenetic evidence are coming down the road. “The problem that you’re going to keep running into, though, is finding statistical significance for second generation or third generation injuries, and linking it back to a particular genetic or epigenetic defect,” he said.
For the Michigan victims of PBB, it’s something of a moot point. In the 1960s, the multinational corporation Velsicol purchased a controlling interest in Michigan Chemical before dissolving the company after the PBB disaster. The plant was eventually torn down, and in a bargain struck with state officials and the Environmental Protection Agency, Velsicol was permitted to escape blame for any human damages outside the plant in exchange for paying the state of Michigan $38.5 million to clean up and construct a concrete cap over the site in St. Louis. To the best of his knowledge, Carl Cranor, a bioethicist at the University of California, Riverside, said no one has yet won a lawsuit on the grounds of multigenerational epigenetic harm, nor is the science yet ready for such a suit.

In a legal case that may well define epigentic tort law, a New York district court judge ruled that “It is our duty to confine liability within manageable limit. Limiting liability to those who ingested the drug or who were exposed to it in utero serves this purpose.”
Taking regulatory action may prove no easier, despite Wachtler’s opinion in the Enright case that it was the FDA’s role to promote safe medications, not the court’s. Although contaminants like PBB fall under the purview of the EPA, the principle remains the same. The problem, according to Mustafa Ali, former senior advisor of environmental justice and community revitalization at the EPA, is that testing chemicals even for acute exposures in single generations is expensive, often costing upwards of $330,000 per compound. And the anti-science, anti-regulatory climate in Washington makes it profoundly unlikely that multigenerational toxicological testing will begin as long as the current administration is in power.
“When you’re trying to develop policy, you need to have strong science in place. Many of the chemicals haven’t been evaluated for that level yet. That’s where one of my great concerns comes in with the cutting of budgets and sort of taking a step back from this needed science,” Ali said.
Still, many experts believe it is only a matter of time before human epigenetic inheritance moves, legitimately, from the murky realm of speculative and uncertain science to something more concrete and culturally — and perhaps even legally —speaking, more consequential. In that sense, should the work of Marcus and other researchers ultimately yield fruit, it can seem impossible to overstate the potential impacts.
The early morning of December 14, 2013 revealed a snowscape in St. Louis, Michigan. Temperatures hovered right around freezing at the town’s four Superfund sites, including the site of the former Michigan Chemical Corporation plant. Murray Borrello and Ed Lorenz, both professors at nearby Alma College were assisting Marcus with a blood draw event at the St. Louis Town Hall. They knew her team was counting on a large turnout to get the samples she needed for her coming years of research. Bad weather was the last thing they needed. By 8 a.m., two hours before the blood draws were scheduled to begin, they realized they had the opposite problem — a line of people wrapped around the building, shivering and stamping their feet to stay warm.
The team ran out of chairs for everyone and by noon, they had run out of needles and Vacutainer tubes for blood. Marcus’s assistants raided local hospitals and health departments for supplies, but even with their scavenging, the Emory team had to turn people away. Nearly 200 people showed up to provide samples and fill out questionnaires. Though he wasn’t part of the initial Michigan Department of Community Health study, Jim Hall was among them — in part because Marcus wanted to cast a wider net, a hunch that paid off. When Hall’s results arrived in the mail several months later, he learned his body still contained massive amounts of PBB — 5.5 parts per billion, 16 times more than many farm families in the 1970s — and fathers like Hall were precisely the individuals Marcus needed for her study.


Because PBB persists in the body’s fat stores potentially indefinitely, children of exposed mothers had direct exposure to PBB in the womb and during breastfeeding. In daughters, egg cells destined to become grandchildren form during fetal development, giving even maternal grandchildren a direct PBB exposure. To prove transgenerational epigenetic inheritance, Marcus would have to wait until the arrival of the great-grandchildren of exposed mothers, the equivalent of Skinner’s F2 rats.
Fathers, however, only provided their DNA. If, like Jim’s wife Ida, the mother was not exposed to PBB (Ida grew up in Middleton, Michigan, 20 miles from the PBB mix-up but has no PBB in her blood), Marcus and Smith could start seeing effects in the grandchildren of these fathers.
“We need three generations, and the first generation has to have an unexposed mom, so that we know that we know that the second generation was not directly exposed in the womb and that they only get the exposure information that’s contained in the father’s epigenome,” Marcus said.
“Since the exposure was in the early 1970s,” she continued, “we know quite a number of families that have three generations, although, it’s going to be difficult to identify families that have that exposure pattern.”
Marcus and Smith are trying to find 20 to 25 of these families, and hope to start a small pilot study by 2019. The hardest part has been finding unexposed mothers, since nearly 90 percent of Michiganders were thought to have been exposed to PBB in the 1970s, and upwards of 80 percent of them still have elevated blood levels of PBB.
Diving more deeply into PBB’s toxic legacy, including finding evidence that could provide conclusive proof about multigenerational epigenetic inheritance, has hit a major roadblock. In the years before the Michigan Department of Community Health handed over control of the study to Marcus, they transferred the paper and electronic study files to the Michigan Public Health Institute (MPHI) for digitization and storage. When individuals signed consent forms to have their information transferred to Emory, MPHI was unable to locate many of them due to missing data. For once, Marcus’s broad smile and easygoing manner slipped from view as she discussed the issue, though she now says that the Michigan Department of Public Health is committed to searching for the additional historic data. Without it, her studies won’t have the critical number of people needed to show the potential multi-generational effects of PBB.
Community members are even more frustrated at the delays from MPHI, which have been hindering research that could finally answer health questions that have loomed over them for nearly half a century. “I think it’s that they don’t care,” Lorenz said bitterly.
Perhaps so, but John Greally, a geneticist at the Albert Einstein College of Medicine in New York suggests that no matter the data and no matter the cohort, proving epigenetic inheritance in humans will be a tall order. It doesn’t help, he adds, that epigenetics has become a scientific buzzword that has different meanings to different people. “We rarely clarify what we mean when we use the word,” he said in an email.
To make her case, Marcus will not only have to show changes in regulation to specific genes caused only by exposure to PBB, her team will ultimately have to outline a mechanism for how those changes lead to disease. The list of variables that can cause changes to DNA methylation seems endless — different cell types have different patterns of regulation, and natural variations in the genetic code can also affect DNA methylation patterns. Cross-sectional studies that use individuals already diagnosed with a specific condition can’t determine whether epigenetic changes caused the disease or are the result of the disease — although researchers are actively exploring ways to do this. Peel back one layer of complexity, Greally said, and you find another — Russian nesting dolls made of As, Ts, Cs, and Gs.
“To my knowledge, scientists have only been able to show multigenerational epigenetic inheritance in animal models, never in humans,” he said. “But that isn’t to say that it can’t or won’t be done.”
In the meantime, families in St. Louis, Michigan struggle to leave because their homes are so close to the old Michigan Chemical plant. Jim Hall can’t get ahead because his thyroid problems and his daughter’s illness drove him into severe debt. As Hall and others see it, the one thing they could give their children — a healthy start in life — was taken from them by a chemical exposure nearly half a century before. No lawsuit or regulation will return these stolen inheritances, but Hall — and Marcus — both said they hope that the research now underway will one day mean that fewer communities are poisoned, and perhaps that fewer genomes will march forward into the future scarred by the exposures of generations past.
“This is our home,” Hall said. “There’s no reason this should have happened, and I don’t know if they’ll ever be able to really clean it up.”
CORRECTION: An earlier version of this piece incorrectly identified one of the organizations that analyzed cattle feed for Frederic Halbert. It was the Wisconsin Alumni Research Foundation (WARF), not the Wisconsin Animal Research Foundation.
Carrie Arnold is a freelance science writer from Virginia. She covers all aspects of the living world and has written for a variety of publications including Mosaic, Aeon, Scientific American, Discover, National Geographic, and Women’s Health.










Comments are automatically closed one year after article publication. Archived comments are below.
This started earlier buy the late 60’s. Farmer’s detected their animals were sick and their babies were deformed. It took until 1973 to release information. Meat and diary products consumed.
I lived in Grand Rapids from 4 yrs old (1969-1984) and was exposed to PBB. I have had three surgeries for endometriosis, I have multiple fatty tumors and so do both my children, and now I am fighting nerve pain. I live in the NW now and the doctors do not know what is causing it. My four brothers and their children have fatty tumors and multiple health issues also. When I mention PBB I get a blank stare and dismissed.
Hi, I was born in 1964 and grew up near the river in Saginaw. We ate the old fashioned meat and potato w milk at every meal. My parents frequented a questionable discount butcher during and after the PBB crisis. I experience menarche at 10.75 years and now have a JAK2 V617F mutation and multiple sclerosis. My daughter was dx with leukemia at age 5 in 1987. I think we were affected by maybe both PBB and the Dioxin. My parents and grandparents do not have health problems like this, though one grandparent was a career worker at Dow.
I remember when growing up in Northern Michigan, my dad bought milk from the local farmer. we continued to buy milk until he lost at least 10 cows, and so did everyone else. I am first generation PBB. My brother had a child 4 years ago, My brothers also are first generation. My nephew has allot of problems, as described in this and other articles, his is underweight for his size, and has intestinal problems…..similar to crohns disease. I have crohns disease as well. But here is the interesting part:: I was 11 years old when contaminated. Less than a year after contamination, I had unusual intestinal developments..
There are so many cases of cancer, intestinal cancer, developmental physical anomalies in northern Michigan for at least 2 generations that I know of, it is unrealistic based on other areas I have lived after growing up. So many generations sick.
And as I can remember, the milk farmer said that it was like months before the CDC could get to northern michigan because of all of the farm cases in southern michigan.
what I am interested in is finding out is how much PBB my nephew (second generation) has in his system. Please feel free to contact me. It is also unclear if my brother’s wife was also contaminated as well. Her mother if so, would be first generation PBB. she would be second.
Also the farmer was told to bury his cattle on his land. (He didn’t have a large enough herd to warrent moving the animals to the “official” dump site.
I have just learned that I am a second generation individual impacted by PBB and having problems outlined by the Emory study. I believe that this company should not be allowed to get away with such an atrocity. I wasn’t alive in 1973 but I am suffering the consequences of their disgusting oversight. There is a special place in hell for the people responsible for this. I won’t be letting this go without a fight.
My family and I lived in Battle Creek from 1973 to November 1976. My son was born in April of 1975 with twisted lower legs that require the use of a metal brace for several months to correct. My daughters 7 and 3 in
1973. My oldest daughter had two still healthy children born in 1996 and 1999. My youngest son just had his first child – a boy – in September of 2017. My youngest daughter never had children but had considerable “female problems”. We are all scattered now from Florida to Alaska to Massachusetts. I would be interested in participating in any follow up studies regarding our exposure and the affects of the continued presence of PBB in our tissues. I have both books and it is my understanding that PBB remains in your tissues for life and can be passed down to future generations.
Actually, make that “global businesses and Departments of Defense.” The United States is hardly the only industrial polluter on the planet. The toxic wastelands of the former Soviet Union put American Superfund sites to shame–stand on the shores of Lake Karachay for one hour and you will die of radiation poisoning.
https://grist.org/article/meet-the-lake-so-polluted-that-spending-an-hour-there-would-kill-you/
I believe history will call this era not just The Industrial Age, but “The Age of Industrial Poisoning.” Which, sadly, is still ongoing.
We have done so much poisoning, from private businesses to the U.S. Defense Department. The mind boggles at how many megatons of lethal chemicals and potions were dumped into our air, land, waterways, and oceans, and that’s only the Flint- and Love Canal-type disasters we know about. Quadruple that for all the hidden and secret polluting, and you see the scope of our crisis more clearly.
Hi Carrie,
I’m a professor at Central Michigan University, working with community members and research partners to develop an oral history project and identify research collections related to this episode of contamination. I wondered if you would be available and/or willing to chat?
Many thanks,
Brittany Fremion
It is important to not trade one problem for another. Chemical exposure has its dangers.
So does starvation. And starvation is more immediate.
ROUND UP – Glyphosate the active ingredient in Monsanto’s herbicide. Hundreds of tests and independent, reliable studies have been conducted that expose Glyphosate as a major threat to human health, but since the FDA and EPA rely solely on safety tests done by the manufacturer, no regulations are put in place to protect humans, animals or the environment from this known cancer-causing weed killer.
We guinea pigs consume ROUNF-UP thru Corn, soy, cotton, sugar, wheat, and other grains. It is unavoidable unless you grow your own food.
We guinea pigs ingest an array of other pesticides and insecticides applied to fruits, veggies, berries, nuts and when we are ripe, the drug companies step in to help us with our pains and diseases.
The fight on cancer is all backwards, the fight should be PREVENTION of cancer. If we tread lightly upon this planet and return the ecological system back to its natural healthy status and eliminate the toxins and chemicals we humans have created, perhaps we could enjoy good health too.
It is clear a negative feedback loop is currently underway, the mental and physical health of our youth is and will continue to be severely compromised by our current farming practices of lacing poisons and toxins on our food , water, air…
I recall driving thru Green Bay Wi, in the front yard was a young dad spraying dandelions with Round up and in the other hand he was carrying a infant boy. Why wait til the kid is 10 or 11 yrs old, teach them young, the result is the same. We are willing participants in an extinction level event, the cause of which is not a great volcano , asteroid or alien or tidal wave, its simply us in Ludicrous mode.
WHAT CAN YOU DO.. Ban all pesticides, herbicides, insecticides in your local communities. Educate your children about these deadly toxic mixes on all your food. Go to your local community and ban application in you village and towns.
Very iportant and well-done article. Hmm. Have to prove in great detsil exactly why and how the defects ocurred. The law doesn’t even require that any drug mfr show exactly how and why a drug works.
Remarkable things here. I am very happy to look your post.
Thanks a lot and I am looking ahead to contact you. Will you please drop me a mail?
Great article that should be viral. Just as the SC resident posted, several people that were exposed moved to other states and are suffering from the generational chemical exposure. I believe this issue is far larger tban to One state. Did anyone trace all the distribution locations prior to the chemical exposure being disclosed? How many millions were exposed consuming the products prior to exposing the problem and once it was discovered? I don’t see where it has been documented the locations that beef, eggs , milk, cheese, etc.. landed while being contaminated before and after discovery. Additionally, killing and burying in the ground, doesn’t this contaminate the soil? Just a few questions I stumbled upon while reading the article.
Thanks for publishing very informative as well as, frightening for those of us 40yrs later.
I was 16 in 1973. My family lived 7 miles from St. Louis. If you are looking for subjects, some of us might be appropriate. There were 8 of us (3 boys and 5 girls) who were (in 1973) between 10 and 20 years old.
This article is very well written. Thank you to the author and to the scientists and researchers who are involved in discovering and publicizing this important information.
I am a lifetime Michigander and would be interested in participating in the study. Born in 1961, near Detroit. I have three male offspring. No grandchildren, yet.
Please let me know any info on whether there is a need for additional study participants.
Makes me sick at heart. Read the entire article and believe it. My grandson was born with a hole in his heart. There is still a small leak and he is also extremely anxious, depressed has other health issues. We grew up in this area mentioned, ate the eggs, meat, milk etc. I have SLE which I have always maintained is environmentally caused. My daughter and son have Reynaud’s. Now we live in SC which has been the dumping site for nuclear waste for years. Why? How stupid and greedy are people?
And there are people in Michigan who vote for the gop. They stand for allowing industry full and complete rights to pollute at will. Some folks are too dumb to tie their shoes. The gop stands for the murder of kids by denying them health care including vaccines. With no herd immunity horrible deadly epidemics are in our future but the gop lovers don’t believe in science so they don’t comprehend the risk. The gop stands for starving thru taking their SS and murdering the elderly by denying them Medicare. But the gop voters are happy to see their parents and grandparents die early so the corporations can save billions on pension costs. Much of Michigan supports the gop with religious fury. Of course many of their religions love the bigoted hate filled woman hating control freak biblical horseshit. This religious myth is too insulate them from their own fear of death, but this does not extend to any others. They are happy to see everyone but the the fetus in the womb murdered with impunity. The children out of the womb can be merrily murdered by the gop and many people in Michigan will still vote for their gop, like an unchangable bad habit without thought or consideration.
I read how these researchers are investigating clues about chemical affects, but I am a human “guinea pig” suffering from intense chemical sensitivities. As soon as I smell these chemicals I suffer reactions. I cannot sleep on conventional mattresses or use laptops. These make me ill, immediately. We do not realize that we daily inhale a barrage of chemicals which Immediately enter our blood, interrupting immunity and hormonal balance. Everyone is suffering from the toxic affects of their many fragrances, formaldehyde in furniture, flooring, cardboard, clothing. But no one perceives it, so we beseech our doctor for a cure, which ensues in imbibing prescribed substances usually unnatural to our bodies. Our bodies just need us to get back to what is natural.
In my reading about epigenetics, I learned about a study, I believe in Denmark or Norway, decades ago that for any woman who had been malnourished in childhood (a nation-wide problem at the time) any of her male children were affected genetically, while any daughters where not although their daughters were.
That would be the Dutch “Hunger Winter” at the end of World War II, which should be enough information to search for much more about it if you like. It is often presented as a case of epigenetic inheritance in humans.
WARF is the acronym for Wisconsin Alumni Research Foundation. NOT Wisconsin Animal Research Foundation. Warfarin is one of the products from this research entity at my alma mater.
Thanks for pointing out this error, Beverly. We’ve updated the story.
You people need to talk to John Berger owner of NWFF in Philomath Oregon. He was a Firefighter that was exposed to some chemical while fighting a fire in California. California fire fighting law was changed due to his case. After he was exposed he would become drunk after drinking orange juice and that was one of the more mundane changes. He has children and they have obvious genetic damage. At this point we are essentially concentrating poisons in the environment. Pollution is the greatest killer at this point and we have affected our genetics probably permanently because of it.
More practical mechanism search involves anti-depressants.
See Dentate Gyrus and BDNF.
Difficult–
Holocaust work remains controversial, involving Fkbp5.
New protocols are emerging.