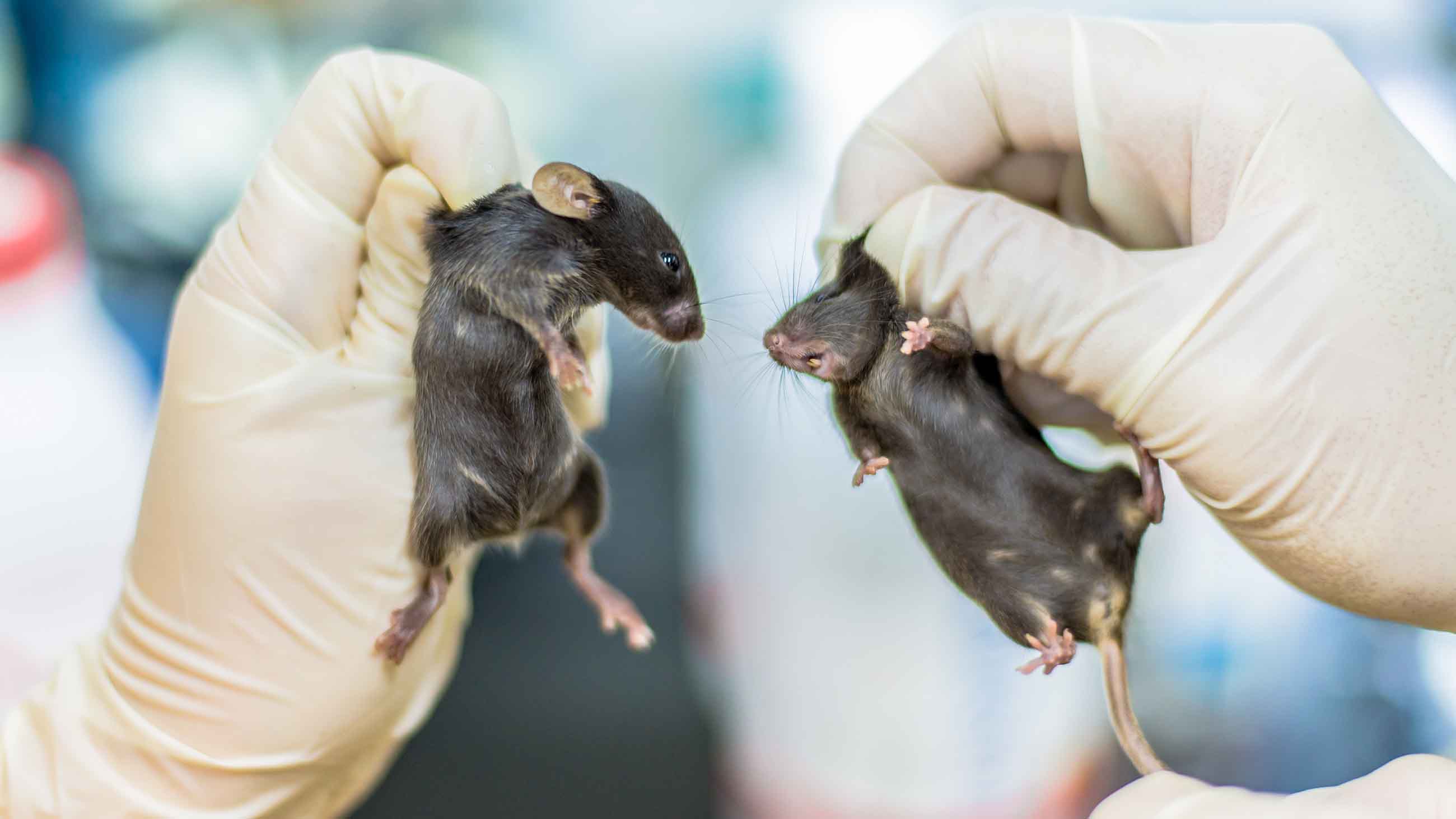
Each year, according to the U.S. Department of Agriculture, roughly 820,800 guinea pigs, dogs, cats, and other animals covered by the Animal Welfare Act are used in research in the U.S.; of those, about 71,370 are subjected to unalleviated pain. These stats don’t track the millions of mice and rats that are used in lab experiments and excluded from the animal protection law (although the rodents are covered by other federal regulations). Scientists and their institutions say they’re committed to keeping pain or distress to a minimum in lab animals where they can. But how do you know how much pain a mouse or a zebrafish feels? And who decides how much pain is too much?

DILEMMAS:
Of science, ethics, and us.
The issue of animal suffering was in the headlines earlier this year, when landlocked Switzerland banned the culinary practice of boiling lobsters alive. No one knows for sure whether these big-clawed crustaceans, equipped with only a rudimentary nervous system, experience pain. Nonetheless, Swiss authorities now require stunning lobsters in a humane way before tossing them into the pot.
I read of this milestone in crustacean rights with bemused fascination and anthropomorphic cringing, as I imagined the lobster’s hypothetical plight. But the Swiss move also made me wonder how scientists measure and deal with animal pain in research studies. Experiments that use critters to simulate human illness or injury are stepping stones to the medical treatments we all use. Yet, the benefits we reap must outweigh the costs to animal welfare for those sacrifices to be justified, ethicists and animal advocates say.
To learn more, I called veterinarian Larry Carbone, director of the animal care and use program at the University of California, San Francisco (UCSF). Policies in most countries call for easing or preventing pain or distress in lab animals whenever possible. Since the animals can’t talk, knowing how much discomfort they’re in to begin with “is really difficult,” Carbone told me. But over the years, researchers have devised good standardized ways to measure reactions to painful stimuli in rodents and other animals. This might, for instance, include timing how fast a mouse yanks its paw away when you shine a hot light on it, and seeing how that reflex differs when a pain drug is administered.
More sophisticated tests track behavioral changes to gauge how much a painful situation bothers a rodent, Carbone said. For example, if mice in pain are given a choice between a chamber where their chow was laced with an analgesic, versus a chamber with regular food, they spend more time in the place that’s associated with pain relief. (Animals respond to many of the same analgesic drugs as we do.)
After rodents have undergone abdominal surgery in an experiment, researchers also can monitor for signs of pain (such as writhing or unsteady walking, or changes in burrowing or nest-building habits.) Mice are highly motivated to build nests, Carbone said, and “we know if they’re in really bad pain, as much as they want a nice nest, they’re not gonna put the work into doing that.”
Still, scientists don’t have ways to measure animal discomfort or distress in all experimental contexts, including those causing chronic pain or anxiety. And it’s been controversial whether certain kinds of creatures — not just lobsters but also fish, whose brains are so different from ours — suffer from pain at all.
While most animals reflexively react to harmful stimuli (think of that hot light), that’s not the same thing as feeling pain and suffering, which are subjective experiences. “We can never know for sure” whether lobsters and fish go through that, said biologist Hanno Würbel, who chairs the animal welfare division at the University of Bern in Switzerland.
Still, he believes that recent studies make “a plausible case” that they do.
By law, research labs in the U.S. are supposed to assume that things that are painful to people are painful to other animals — including fish. The task of then deciding how much pain may be inflicted in experiments falls to a committee that oversees the care and use of animals at each research institution in accordance with the Animal Welfare Act and U.S. Public Health Service regulations and guidelines. These local committees approve or reject proposed study protocols, reviewing whether the pain or suffering in animals would exceed acceptable limits, or could be allayed by anesthesia or analgesics.
Every case is different, said bioethicist Tom Beauchamp of Georgetown University. “There’s a huge range of territory for people to disagree over these matters, which is why there’s so much disagreement about the justifiability of laboratory animal research to begin with,” he told me. Compared to the U.S., regulations in the European Union are tougher, requiring a formal, detailed analysis of whether the expected harms to animals in an experiment are justified by the anticipated societal benefits of the research. Animal advocates such as Cathy Liss, president of the Animal Welfare Institute, point out that the U.S. has long been “out of step” with many other countries in failing to include lab rodents under the protective requirements of the Animal Welfare Act.
But on either side of the Atlantic, the bottom-line is that U.S. and European policies do allow for experiments that cause severe, unalleviated pain to animals when it’s the only way to gather valuable scientific data.
The dilemma, Carbone told me, is that some experiments — testing a new drug designed to treat the agony of bone cancer against a placebo, for example — are difficult to do without inflicting pain. In other cases, scientists have legitimate concerns that using painkillers to treat discomfort caused by experimental procedures could skew the results. A study using rodents to assess whether an infusion of stem cells could help patients after a heart attack could be one such instance, Carbone noted. Dosing the rodents with pain relievers after they’ve undergone a surgical procedure that simulates a heart attack might affect their response to the stem-cell therapy.
On the other hand, he pointed out, pain itself can trigger immune system responses, hinder sleep and eating patterns, and possibly impede postsurgical healing — so if you don’t treat the animals’ pain, it might also skew the experiment’s outcomes. So is the quality of a research study better when pain is relieved, or not?
It can be a real ethical and scientific conundrum, Carbone said, often with no clear answers. Maybe it turns out that to get usable data on a particular question, untreated pain is unavoidable. “But then, the ethical question is, Okay, is that worth it?” he said. “Do we really need this? Is every single scientific question important enough to subject animals to pain?”
From Liss’s perch at the Animal Welfare Institute, any study that looks to use animals warrants careful consideration, she said, but “there should be an incredibly high bar if any individual animal is caused unrelieved pain.”
In a report this March from the University of Bristol in the U.K., medical sociologist Pandora Pound and animal welfare researcher Christine Nicol provided a big-picture perspective on whether animal research is worth it. In the first study of its kind, they systematically assessed the harms versus benefits of 212 animal studies relating to six drug therapies, four of which are in clinical use today. Conducted from 1967 to 2005, those studies collectively used around 27,149 mice, rats, pigs, sheep, monkeys, and other animals.
Most of the protocols appeared to inflict severe harms on the animals, with 13 percent of the studies failing to report use of anesthesia and 97 percent making no mention of pain-relieving drugs. Overall, they found, the studies were poorly designed, which meant they couldn’t contribute conclusive findings toward clinical benefit. In such cases, “any suffering endured by animals loses its moral justification,” Pound and Nicol wrote in an email to me.
Using a cost-benefit analysis tool to assess the ethical acceptability of the studies, Pound and Nicol deemed that more than 93 percent failed to pass muster. “At present the situation is deeply unethical,” they concluded, adding that the general argument that harms of animal research are justified by benefits to people “does not hold up.”
These are sobering and contentious charges, and Pound has previously drawn criticism for her views. But there are important caveats to this latest work. For starters, it’s always hard to predict the ultimate benefits from any animal study, especially with basic research that investigates how biology works.
Further, while almost none of the studies in the analysis reported using analgesic drugs, we don’t know for sure how often animal pain actually went untreated. In a 2016 study, Carbone and a UCSF colleague found that 40 percent of animal studies involving major surgery failed to mention using anesthesia — even though such surgeries usually do employ it, Carbone told me, because otherwise a mouse or rat won’t stay still for it. Many researchers just don’t bother including the information in their write-ups.
At the same time, around 75 percent of studies didn’t mention giving pain relievers, also suggesting that post-surgical discomfort is probably undertreated in animals. But without detailed data, the UCSF researchers noted, it’s hard to draw firm conclusions. Carbone is hopeful that things are getting better: More and more, he told me, it’s becoming standard for lab veterinarians to give rodents long-lasting pain drugs after surgery.
The authors of the newer U.K. report acknowledged that some animal severity ratings in their analysis may have been lower if anesthesia and painkillers were indeed administered — although Pound noted that those ratings were only one of the factors weighing into the overall harm-benefit analysis results. For now, they’re sticking with their conclusions and recommending reforms, including adoption of technological alternatives to animal research.
Würbel, the Swiss biologist, called for a more measured reading of things, however. The U.K. study is innovative, he said, but it’s one “in a whole ocean” of meta-research parsing the reliability and value of animal studies. “I think if you look at everything, then you will get a more nuanced picture of the situation,” he said. “I think we have learned a great deal from animal research.”
Meanwhile, when I asked the NIH Office of Laboratory Animal Welfare for its take on the report, a spokesman (in the Office of Extramural Research) sent me a five-page email response that explained the laws and policies that govern NIH-funded experiments on lab animals. To cut to the chase: The agency takes the humane use and care of research animals “very seriously.” It pointed out that the U.K. analysis reviewed an older body of studies, which don’t reflect today’s U.S. standards for the use of lab animals. And it emphasized that animal research helped pave the way for treatments for many devastating conditions in people and “continues to revolutionize our understanding of health and disease.”
Okay. Yet it also seems clear that current practices involving animal experiments remain far from perfect. As Würbel put it, “there is huge scope for improvement” — including a pressing need to address the problem of sloppily conducted animal research whose results fail to hold up in studies of people.
If studies don’t produce valid, reproducible results, he said, “you’re simply wasting animals for no good reason.”
Ingfei Chen is a California-based writer whose stories have appeared in publications including Scientific American, The New York Times, and Spectrum. She is a former Knight Science Journalism fellow at MIT.
Email us at [email protected] if you’d like to seek input on a quandary of your own. Undark will talk to experts on ethics, philosophy, or standards of ethical scientific or journalistic practice and share their best wisdom on possible solutions. For those wishing anonymity, we’ll withhold your name from any resulting Q&A items that we publish.










Comments are automatically closed one year after article publication. Archived comments are below.
The entire article begs the question whether or not any of those animals should be used in the first place for scientific research. These animals do not consent to the research, did not volunteer and they want to live as much as you and I. Leave them alone
I understand why people feel that it is wrong to test on animals. But at the same time I find it incredibly stupid to complain about something that you aren’t willing to change. I see a lot of people who do not support testing on animals but also wouldn’t sacrifice their bodies to testing. If you don’t wanna test on animals anymore then sign yourself up to be tested on. The things that are tested on animals are usually things that are trying to benefit humans. If you cared enough about animals you would volunteer to be the lab rat.
As a purely scientific concern that might also help the ethics/philosophy side: Certainly if there’s an interaction between the study drug and/or standard drug and painkillers, we would want to know if it occurs in humans as well as the animal model.
A model should be as realistic as possible. There is no realistic scenario in a well-run healthcare setting where a human with an extremely painful condition would not be given painkillers for symptomatic relief as well as the targeted therapy. So if an animal model is being inflicted with a condition where a human would be given painkillers, it could also make sense to give the animal model treatment and placebo groups equivalent doses of painkillers.
Many promising preclinical drug candidates have been scuppered by interactions with real-world interactions in humans that weren’t accounted for in in vitro or animal models. Painkillers could definitely be one to look at.
The problem is deep. Modern Allopathic medicine itself needs to change. It treats, not only animals, but even human patients as ‘things’. Only by mid-20th century, terms such as “holistic medicine”, “mind-body medicine” started to emerge, but continue to remain non-mainstream ideas. No alternative medicine system that I know of involves any animal testing. Declaring all animal testing in pharmaceutical industry will force Allopathy meficine to seek a theoretical basis for its very conception of what counts as a living body. Medivine is supposed to save lives, but the very notion of life remains scientifically undefined in medicine, all they can do is to try save bodies, not life. A body in modern scuence is mere res extensa, extended substance, a la Descartes.
An implicit assumption of animal research that human lives are by definition superior to other forms of life, without an explanation of why it is so. Other forms of life are referred to as “lower” forms of life, and may be so at the level of body, but is “life itself”, as biologist Rosen called it, across bodies lower or higher? We may all agrre that cycles and motor cycles are (mechanically soeaking), lower forms of transport compared to car. But can we thereby say the living principle in each of these vehicles, the drivers, are also lower?
Animal researchers should be asked to consider the possibility of karmic reaction, that they would have to be also born as the rodents etc., and receive similar pain meted out to them. Of course, the cycle feeds itself and can become only more viscious. Animal research must therefore stop at all levels forthwith, regardless of the financial cost to companies involved. If relieving you pain requires some othe even more pain, how far it can be justified?
They are animals. They need the normal, calm life. Need the petition signs. Don’t pain in lab animals. :(
Any type of pain is too much. It is animal torture, pure and simple. Vivisection must end immediately.
This is terrible abuse.. PLEASE stop the torture.. you were made to have compassion for animals, they are not just THINGS . They are living creatures.. NOT put in this world for anyone to treat them this way.. they are not ours!! People that can perform such acts are a disgrace to the human race.. with no feelings or comapssion..sad.. there will be a day that you will answer for the pain tou have caused, all for the sake of profit.. shameful
It should be noted that nearly all University Animal Care & Use Committees are closed to public scrutiny. As taxpayer-supported institutions, they should be REQUIRED to have public access, thereby curbing some of the most outrageous abuses. The public has a RIGHT to know.
I’m ever mindful of a line in I.B. Singer’s book, “Enemies: A Love Story.” The novel’s protagonist, returning from a slaughterhouse, muses to himself, “As far as the animals are concerned, all men are Nazis.” Keep in mind that Singer was a Holocaust survivor.
Homo sapiens (sic) is truly a cancer upon the planet, to the detriment of all other life forms. Non-living forms, too, a huge evolutionary mistake.
Vivisection is barbaric and morally disgusting. I once saw a picture of an animal who was so covered in lesions and tumors that I could not identify the species. Animal experimentation, where living creatures have no power to consent to such painful exploitation, has no place in an advanced society.
Unfortunately, too many believe that the most horrid abuse is justified if human beings are benefited. I hope future generations will think differently.
Please stop
Any is to much. They are live animals.
Stop the heinous torture of sentient beings.
Being human does not grant you the right to do so.
By participating in inflicting such pain on innocent
defenseless creatures, your vibration is lowered
to the most base and cruel of species…unevolved
humans.
Let them go. Testing on animals is horribly wrong! God did not create them for this, you are NOT God. May your karma return to you in exact same measure.
I appreciate that Ingfei Chen has attempted to shed light on the hidden issue of animal suffering in scientific experimentation. However, as a veterinarian, I must take issue with some of the statements regarding animal pain in this article. In particular, the assertion that “most animals reflexively react to harmful stimuli…that’s not the same thing as feeling pain and suffering, which are subjective experiences” is simply not supported by scientific literature. It is well-established that vertebrate animals (which includes mice and rats) suffer from pain, and experience both psychological and physical effects of acute and chronic pain. Our understanding of invertebrate pain, to include lobsters, crayfish, crabs, and octopuses, has evolved greatly within the past decade, and invertebrates have demonstrated both “motivational tradeoff” and “conditioned place avoidance.” These are measurable indicators of pain that cannot be explained away as a reflex. The argument that we can’t attribute the experience of pain to animals due to its subjectivity is nonsensical. Even among humans, perceptions of pain, “the experience of pain,” differs, and one person can truly never understand another person’s pain. If the argument of subjectivity is taken to its logical conclusion, then we can’t really know that other humans experience pain. Yet experimenters seem to demand this excessive burden of proof, despite the already existing evidence, to escape the fact that animals suffer in painful experiments that do not yield useable results. It is, truly, wasting lives “for no good reason.” Knowingly inflicting pain in the hopes of some vague future benefit (which decades of experiments in animals have shown does not pan out) can no longer be justified. It’s time for biomedical science to evolve.
Thank you, Ingfei Chen, for reporting on this important topic and for including input from Drs. Pound, Nicol, and Beauchamp, as well as AWI’s Cathy Liss. As you mention, no U.S. or European policies forbid experimenters from causing animals ‘severe, unalleviated pain,’ but this does not extend only to experiments aimed to gather ‘valuable scientific data.’ Experimenters can receive permission to inflict immense pain and suffering on animals with even the most hollow justification. During my own experience in animal experimentation, I saw this demonstrated time and time again. You also state, ‘Experiments that use critters to simulate human illness or injury are stepping stones to the medical treatments we all use;’ however, the data disagrees. Ninety-five percent of drugs that were developed using and that test safe and effective in animals go on to fail in human clinical trials because they’re not effective or they’re dangerous. Animal experiments are stepping stones to irreversibly wasted intellectual and financial resources. This is why, increasingly, pharmas and other institutions whose bottom lines depend on success are giving up animal experiments in favor of human-relevant technologies, such as microfluidics (organs-on-chips). Taxpayers who finance federally-funded experiments and those who donate to charities, not to mention patients and their families waiting on cures, also deserve a better return on their investments.
Adding to the complexity is the fact that rodents are prey species and would hide their pain and discomfort. By the time you notice the symptoms, they’re REALLY suffering. We also often see in inspection reports that experimenters fail to give animals pain relief or use expired drugs. It boils down to apathy, which is an acquired attitude that experimenters practice throughout training. You can’t be too bothered by the animals’ suffering, or the day-to-day guilt associated with tormenting animals would eat you alive. How can these same people then be entrusted to carry out animal welfare measures?
In average more than 95 percent of time preclinical animal tests don’t yield human-relevant results. No matter how well cared for the animals are (before being killed), more than 95 percent of time it’s entirely pointless.
Vivisection is horse ‘n’ buggy research. Time for a new path – for animals AND people. Live animal research should be against the law everywhere.
Testing on animals does. not. work. Yet we cling to this cruel practice instead of venturing into new, innovative and truly with the potential for a breakthrough. What’s that saying, “doing the same thing over and over and expecting a different result is the definition of insanity.” Conducting experiments on animals is insanity.
ANY pain in lab animals is too much. ALL invasive research on animals should be outlawed. It’s a true “Crime Against Nature.” And as G.B. Shaw famously wrote, “Anyone who doesn’t hesitate to vivisect, will not hesitate to lie about it.” Such research is cruel, unethical and immoral. It needs to stop. In the interim, follow the money.