A Month in the Life of a Stem Cell Lab

Pluripotent stem cells have the potential to generate any human cell type and researchers have shown that they may be used to repair damaged tissues and organs in the body. But what looks good in the lab doesn’t always translate to the clinic. In laboratories around the world, thousands of scientists are trying to close the gap between promise and real-world therapies.
For a month and a half, starting in January, I was embedded in the daily life of one such group of scientists — Douglas A. Melton’s laboratory at the Harvard Stem Cell Institute. I watched their experiments, learned about the complex science of stem cells, and talked with the researchers about their work and hopes. I was allowed to take pictures, and for this photo essay I tried to pick out moments and details that I found revealing, although scientists may see them as business as usual.
Melton’s lab focuses on diabetes, a disease that affects almost one in 10 Americans. In the lifelong form of the disease (known as type 1), the body’s immune system attacks and destroys the beta cells that produce insulin in the pancreas. Diabetic patients must rely on daily injections of insulin to control the level of sugar in their blood.
Melton’s team has invented a protocol to turn embryonic stem cells into beta cells and has shown that they effectively secrete insulin when transplanted into diabetic mice. To push the research forward, Melton has co-founded a biotech company, Semma Therapeutics, and hopes to start a clinical trial in the next three or four years.
He is not alone in the race. Timothy Kieffer’s lab, in Vancouver, British Columbia, has developed another protocol to turn stem cells into beta cells that reverse diabetes in mice, and the approach is being tested by California biotech company ViaCyte. Big pharmaceutical companies are on the lookout too. AstraZeneca collaborates with Melton’s lab, and the Danish company Novo Nordisk, the worldwide leader in diabetes drugs, is working on its own project.
All have major challenges to overcome before any stem cell therapy hits the market. How results in mice translate into humans is not clear, and finding ways to trick the body’s defense system will be a huge step. New tools such as the gene-editing technology CRISPR-Cas 9 and the ability to handle huge sets of data will help, but they also raise many new questions. “The idea that we can have some mastery and control of the cells is a fantastic thing,” Melton told me, adding that while in the 20th century humans gained control over much of their physical environment, “we are now entering a century when we are going to get control over the human body.”
Chloé Hoorman, a 2016-17 Knight Science Journalism fellow, is a Paris-based journalist with Le Monde, specializing in the pharmaceutical and life sciences industries.
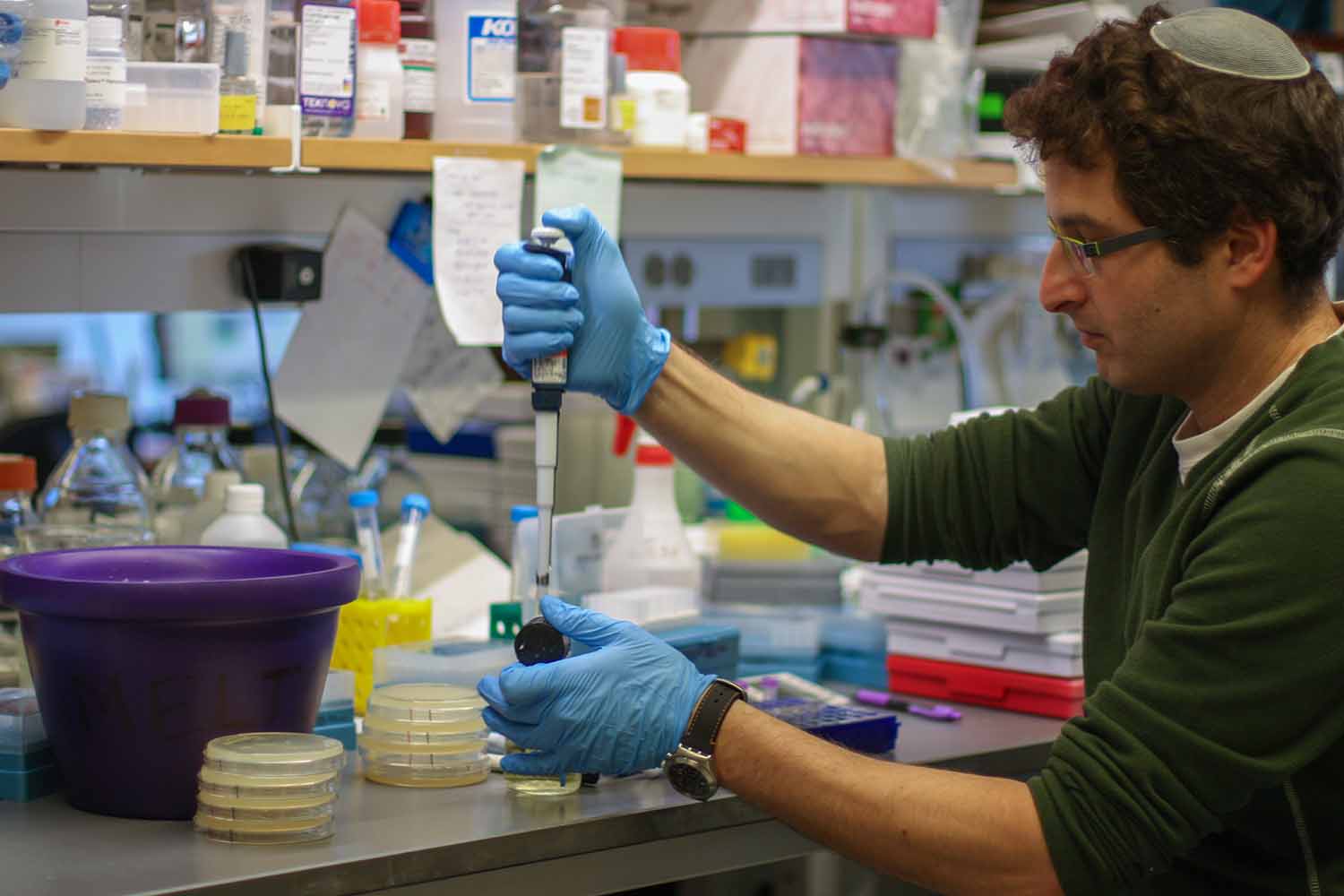
“You Have to Keep the Cells Happy”
Precision is critical in the lab, lest cells become damaged or experiments rendered uncertain.
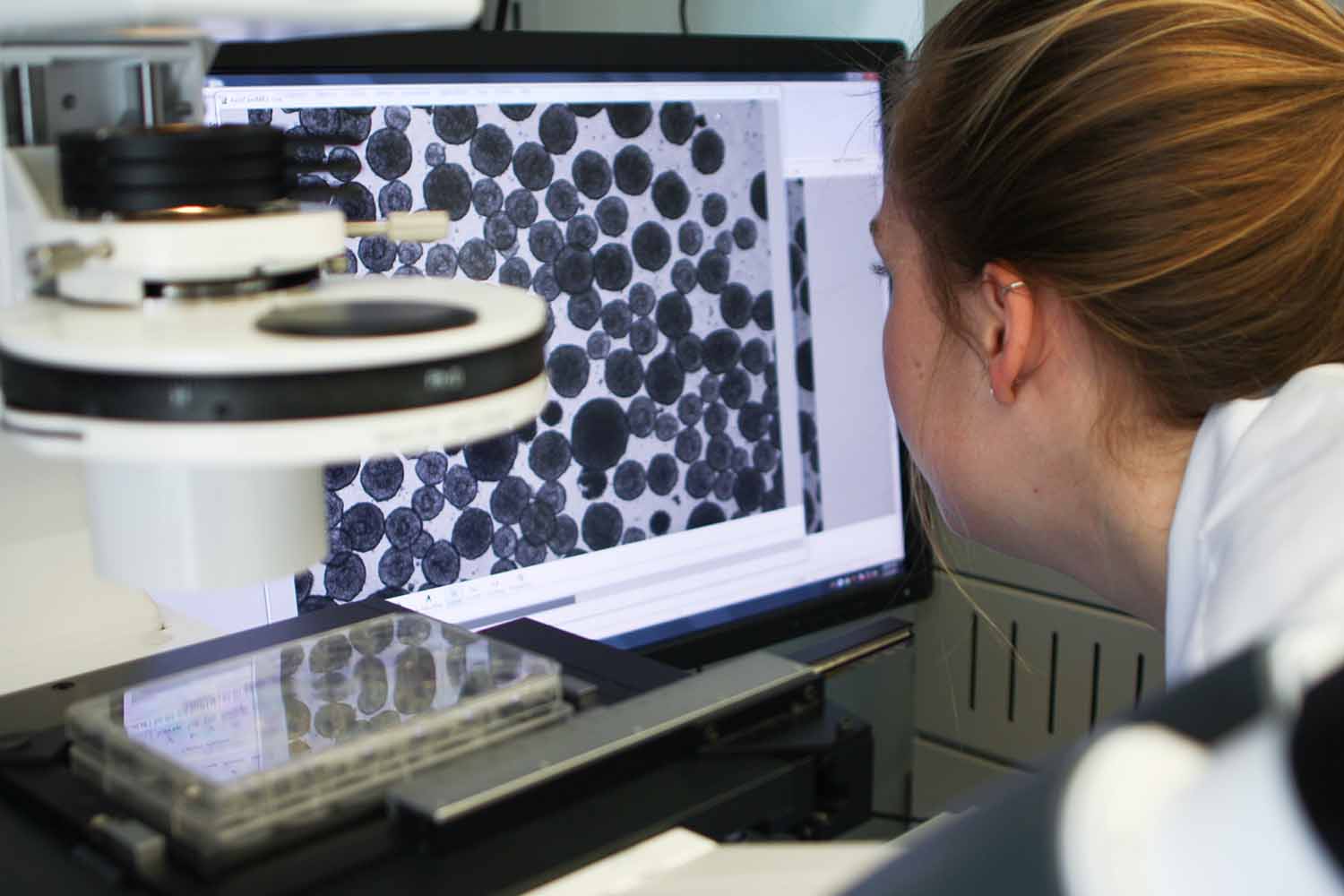



“They Are Like Our Babies”
Sophisticated experiments still require some day-to-day innovation inside the lab.


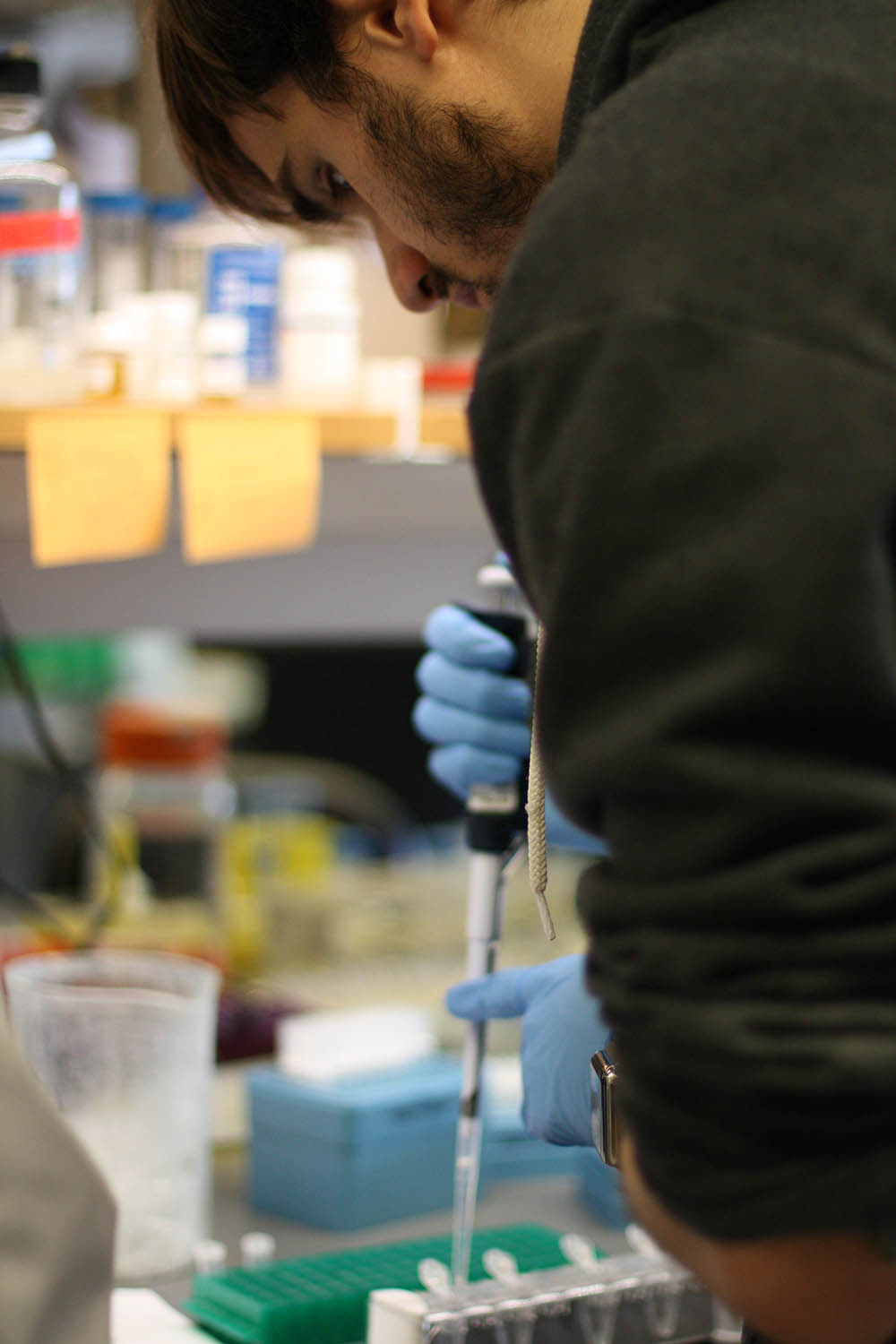
“Biology Has Taken a Turn”
But even with sophisticated genome editing tools such as CRISPR-Cas 9, this is not an exact science.













Comments are automatically closed one year after article publication. Archived comments are below.
Why are none of these people wearing lab coats?