Spilled Milk
The air inside the barn still has an electric tingle, the atmospheric static that lingers after a spring storm. Three females have just given birth to ten kids, and a supervisor for this dairy goat research facility at the University of California, Davis is helping student interns towel off the newborns and carry them, knobby-kneed and sleepy-eyed, upstairs. Wearing a lab coat and nitrile gloves, the technician cuts off two small pieces of umbilical cord from the first goat and places each one in a weigh boat. She then grabs notching pliers and takes a sample from the young goat’s ear. She puts each sample into a microcentrifuge tube before repeating the process with the remaining kids.
Later, the tissue samples will be picked up by someone from a lab overseen by Elizabeth Maga, an adjunct professor in the University’s Department of Animal Science, where they will incubate overnight. The next day her students will isolate and amplify a portion of the goat’s DNA that scientists at Davis have genetically altered to code for human lysozyme, an antimicrobial enzyme commonly found in people’s tears, saliva, and breast milk. Lysozymes work on the front lines of the immune system, destroying bacterial cells that cause diarrhea and other infections.
Not long ago, what Maga and her colleagues were doing seemed revolutionary, given diarrhea’s enormous global toll. According to the World Health Organization, 525,000 children under five died last year from diarrheal diseases, mostly in poor communities in developing nations where waterborne diseases are rampant and vaccines and antibiotic treatments are difficult to acquire and distribute. That’s more childhood deaths than AIDS, malaria, and measles combined.
Maga’s team was convinced that their work had the potential to save some of those children’s lives — and over the course of nearly two decades, they worked tirelessly to demonstrate that the milk from their goats was both safe and effective, earning eager support from UC Davis and a grant from the Bill and Melinda Gates Foundation.
They now have the data to prove it many times over, Maga and her colleagues say. But the world, it would seem, isn’t ready for it.

Instead, the researchers have run headlong into the pitched, prolonged, and, critics would argue, misguided first-world debate over genetically modified organisms, or GMOs. It’s a conflict animated in large part by a rapidly evolving arsenal of genetic engineering tools and the inability of both policymakers and the public to quickly and effectively make sense of it all — or even to apprehend the full spectrum of motivations for manipulating genes, from the mercenary and commercial to the humanitarian. This has left the UC Davis goats, along with a host of other transgenic animals with the potential to curb disease and save lives, in a regulatory limbo — even as other genetically engineered organisms, from corn to fish, earn regulatory approval.
“Regulation is important, and looking at these things carefully is necessary,” says Alison Van Eenennaam, an animal geneticist who works with the UC Davis team and has been outspoken about the scientific community’s frustrations with the regulatory apparatus. “But not at the exclusion of all innovation, ever.”
Particularly not those innovations, Van Eenennaam adds, that might well prevent millions of unnecessary deaths by diarrhea.
The gene of interest to the UC Davis team is called HLZ, and it is a relatively recent addition to the goat genome. Maga and her colleagues at Davis successfully introduced it roughly 20 years ago, through a process called pronuclear microinjection. The result was Artemis, born in this same barn on a spring morning in 1999. She had a streak of black on her hind flanks, bright eyes, and something no goat had ever had: the ability to produce human lysozyme in her milk.

RELATED STORY: Regulating biological innovations has turned out to be a complicated affair — particularly because the policies that would govern advanced biotech were developed a long time ago.
Since then, the human gene in her DNA has been passed on through generations of goats who have spent their lives in the Davis herd, down to some of the ten kids just born. Because of Mendelian inheritance, they won’t all have the gene, which carries with it both tremendous potential to do good and the stigma that comes with the term GMO.
In a day or so, DNA analysis will tell the Davis researchers which of the animals are in effect part human — walking, bleating embodiments of one of the most divisive scientific controversies of our time.
But for the next 24 hours at least, they’re all just goats.
It has been four decades since the first genetically engineered organisms were developed, yet thousands of safety studies later, GMOs remain among the most polarizing scientific issues of our time. Today, the safety of genetically engineered foods represents the biggest gap between public opinion and scientific consensus in America, more so than evolution, vaccines, and global warming. According to a recent survey by the Pew Research Center in collaboration with the American Association for the Advancement of Science, 88 percent of scientists believe these foods are safe to eat. Only 37 percent of the general public agrees. Republicans and Democrats are just as likely to be opposed to transgenic foods, as are people across different age groups. So why is it that we trust the National Academy of Sciences and the WHO when they say climate change is likely caused by humans, but not when they say these foods are safe?
Pam Ronald believes that oversimplification and generalization have undermined public understanding of science. “The major obstacle to discussion at all is the term GMO; it’s meaningless,” she says. “[It] means something different to everyone.”
Ronald is a plant geneticist at UC Davis, where she has successfully engineered rice to tolerate prolonged flooding, common in many parts of the world where rice is a dietary staple. She is also co-author (with her husband, the prominent organic farmer Raoul Adamchak) of “Tomorrow’s Table: Organic Farming, Genetics, and the Future of Food,” which advocates a food system that is organic and genetically engineered. She says there are all kinds of organisms made with all kinds of genetic technologies — some controlled by big corporations, and some, like hers, owned by public universities and given away free. But all are lumped together by GMO opponents. “The majority of consumers want to base their decisions on good information, but there’s always a vocal non-science-based community that wants to scare people,” she says.
Ronald isn’t alone in wanting the public to stop obsessing over whether something is a GMO or not. Last month, the National Academies of Sciences, Engineering, and Medicine released a report assessing all the science available on genetically engineered crops. It concluded that we shouldn’t be making generalizations about GMOs, but rather asking if a particular crop or GE product makes the world a better place or a more dangerous one, on a case-by-case basis. This was not exactly what people wanted to hear, the authors wrote: “We received impassioned requests to give the public a simple, general, authoritative answer about GE crops. Given the complexity of GE issues, we did not see that as appropriate.”
 A new report from the National Academies of Sciences, Engineering and Medicine suggested that generalizations about GMOs are wrongheaded.
A new report from the National Academies of Sciences, Engineering and Medicine suggested that generalizations about GMOs are wrongheaded.Nathanael Johnson, a journalist and food writer for the website Grist, has suggested that if governments were to take this “stop generalizing” appeal seriously, it could have a significant impact on the way GMOs are regulated. Virtually unchanged since its adoption in 1986, the U.S. Coordinated Framework for the Regulation of Biotechnology determines oversight of genetically engineered animals, plants, and derived products. Under its guidelines, regulatory evaluation of a genetically modified product is supposed to be determined by the final product, and nominally agnostic of the technology that made it. The idea behind product-led regulation was to limit the need for new biotechnology laws. Engineered organisms could be channeled to particular agencies (EPA, FDA, USDA), depending on what category they fell into, and governed by existing laws and policies. In reality, though, agricultural scientists have encountered a regulatory system based not on the product itself but on the method used to create the genetic change in it. The factors that trigger regulatory review comprise a specific set of biotechnologies, notably including recombinant DNA techniques (putting a gene from one organism into the DNA of another).
The FDA claims regulatory authority over genetically engineered animals under the new animal drug provisions of the Federal Food, Drug, and Cosmetic Act, requiring a pre-market review. Other genetically engineered products might fall in the USDA’s jurisdiction under the Federal Plant Protection Act, or with the EPA under the Federal Insecticide, Fungicide, and Rodenticide Act. Sometimes more than one law applies and more than one agency is involved.
The resulting regulatory framework, many scientists argue, is outdated, overly broad, unduly burdensome and, for small companies and public institutions, prohibitively expensive. Take the case of the AquAdvantage salmon, the first genetically engineered animal to be approved by the FDA for food purposes. It was developed back in 1989 using technology under license from the University of California at Berkeley. Scientists took a growth hormone gene from Chinook salmon and inserted it into a fertilized Atlantic salmon egg, sandwiched between regulatory gene sequences from an ocean pout. The pout’s promoter sequences kept the growth hormone turned on year round. (Atlantic salmon normally stop growing in winter.)
The AquAdvantage salmon grows about twice as fast as its conventionally farmed counterpart on 25 percent less feed. The company was incorporated in 1991 and applied for regulatory oversight to commercialization in 1995. Twenty years and $85 million later, it was finally approved in November 2015. But the process nearly bankrupted its developer, AquaBounty Technologies, a small biotech company in Maynard, Massachusetts, and it was saved only by cash injections from a Georgian oligarch and, later, a buyout by a synthetic biology firm, Intrexon. And even now, with legal and other obstacles still to overcome, it’s still unclear whether the AquAdvantage salmon will ever wind up on a dinner plate.

It’s because of the immense resources required to get such products to market that companies like Monsanto have become the face of genetic engineering. And it’s the perceived gap between the promise and the fruits of the technology — instead of curing disease and feeding the world’s poor, it has mainly enriched a handful of drug and seed companies — that is at the center of genetic engineering’s 30-year public relations crisis.
Maga didn’t set out to play God. She came to Davis in 1988 to work with lysozymes for food science applications — extending the shelf life of milk and other dairy products. But when her adviser fell ill and had to retire, she became a Ph.D. student without a home. Jim Murray, an animal scientist who worked on improving the properties of milk for human consumption, had been working with transgenics and offered Maga a spot in his lab. They started with mice, showing that those with transgenic HLZ produced milk that slowed the growth of bacteria.
Lysozyme occurs naturally in the milk of all mammals, but it’s especially concentrated in human breast milk: 1,600 to 3,000 times the amount found in livestock milk. Murray and Maga hypothesized that if they could engineer goats to make extra HLZ, they could give the milk to non-nursing infants and young children at risk for diarrhea in effect, restoring the protective effects of breast milk. With a grant from the UC system they developed Artemis, and built a herd from her progeny. In the spring of 2004, once they had female goats producing the extra lysozyme, they started looking at the milk’s effects on pigs (which are closer to humans than mice). The results were immediate: The milk changed the type of bacteria in the pigs’ guts, significantly reducing disease-causing pathogens. Their intestines also looked healthier, with more surface area for better nutrient absorption. Maga was elated. “I thought, ‘Wow, this might really work!’” she says, remembering the results.
Encouraged, she won a grant from the Bill & Melinda Gates Foundation to test the milk’s efficacy on malnourished pigs. Malnutrition and intestinal infections trap millions of children around the world in a vicious cycle, as lack of proper nourishment damages their intestines, leaving them vulnerable to infections that in turn can impair their ability to absorb nutrients.
Maga inoculated pigs that mimicked these physical features with an E. coli infection, then fed some of them HLZ goat milk. Those pigs recovered remarkably faster, with less damage to their intestines.
She was triumphant. Here it was, a highly effective way to combat a major cause of childhood mortality, and all you needed was a very special goat. The Gates grant also offered successful projects the opportunity to receive follow-up funding to further develop the concept. So she applied. Her data was strong, the results compelling. It was exactly the sort of thing the Gates Foundation would be interested in backing — a low-cost, scalable remedy for a public health problem with huge implications for populations in developing nations.
Her application for funding was rejected. In the email she received in April 2014, the foundation provided some mild scientific critique, along with the suggestion that the hurdles in public acceptance of GMOs were simply too high to warrant the testing of “only a single human antimicrobial protein for diarrhea prevention.”
The foundation did not respond to several messages seeking comment, but Van Eenennaam says she is particularly dismayed that the first transgenic animal to win regulatory approval is a growth-enhanced salmon, while the UC Davis goats languish.
“It falls right into people’s worst assumptions about how this technology can be used,” she says.
Instead, she likes talking about projects that can serve a public good, like the lysozyme goats, and then asking listeners whether they are still opposed to genetic engineering. A dozen such projects have been living in regulatory limbo since the mid-90s.

In addition to Maga and Murray’s goats, there are chickens, developed by a research team in Great Britain, that can’t infect other birds with avian flu. A team of Canadian scientists created the “Enviropig,” which produced 60 percent less phosphorus — one of the biggest pollutants from pork production — by adding an enzyme to their saliva. Money ran out and the experiment was terminated, but the pigs’ genetic information remains cryogenically frozen in hopes that the regulatory climate will warm someday.
And then there are the two cows that produce the human enzyme lactoferrin, which has antibacterial capabilities — particularly in human infants. Murray adopted the cows at UC Davis after the demise of the Dutch company that first created them, and they, along with the chickens and pigs and goats, are what he calls “Generation 1.0” animals. They were made with the somewhat clumsy, inefficient recombinant DNA technologies available in the 1990s and early 2000s by scientists with high aspirations for genetic engineering.
Back then, these researchers would gather at the biennial TARC (Transgenic Animal Research Conference) at Lake Tahoe, where they would present papers on their findings and spend afternoons floating on inner tubes down the Truckee River, toasting each other’s successes with cans of cold beer and talking about the better tomorrow they were building together. But like the drought that would dry up the Truckee, so too did funding for transgenic livestock, and with it hope for that bright future. In the early 2000s the USDA stopped accepting grant proposals for transgenic animals.
“If you look between 2005 and 2012 there were no new applications…They just flat didn’t do it,” says Murray, whose 2011 request to have his and Maga’s goat milk approved under GRAS status (the initials stand for “generally recognized as safe”) is still awaiting a verdict from the FDA. And there was a real cost. “Young people aren’t going into [the field],” Murray says, “because they can’t see how they can make a career.”
During the early 2000s, as research for transgenic livestock floundered, pharmaceutical applications involving the same science took off in a big way. Guidelines published in 2008 said the FDA had the right to exercise “enforcement discretion” over some but not all genetically engineered animals based on their potential risk. As a result, the agency decided to not require premarket approval for engineered lab animals used for research.
Today, the biotech drug industry is a multibillion-dollar business built on the backs of millions of genetically engineered animals. Transgenic mice and rabbits expressing human genes have been used to develop and test treatments for everything from AIDS and cancer to heart disease and Alzheimer’s. Genetically modified zebrafish are helping to unlock the secrets of the human microbiome. There are knockout pigs destined for xenotransplantation: a not so distant future where livers and hearts could come not from deceased donors, but from gene-edited animal hosts. All of these animals have escaped the anti-GMO advocates’ bullhorns (if not those of animal-cruelty organizations).
Murray thinks the regulatory process has taken cues from this idiosyncrasy. When he and Maga first applied for approval, they asked the FDA to rule on whether the goat milk was safe to drink, not whether it was effective as an antidiarrheal drug treatment. Their studies had shown that the lysozyme had a bigger impact when consumed as part of milk, rather than in purified form as a diet supplement. “Every human in the world eats lysozyme every day of their life,” he says. “It’s clearly not an allergen and it’s clearly not toxic. … In fact, I thought it would be easier to get regulated.” He pauses. “That turned out not to have worked out as I hoped.”
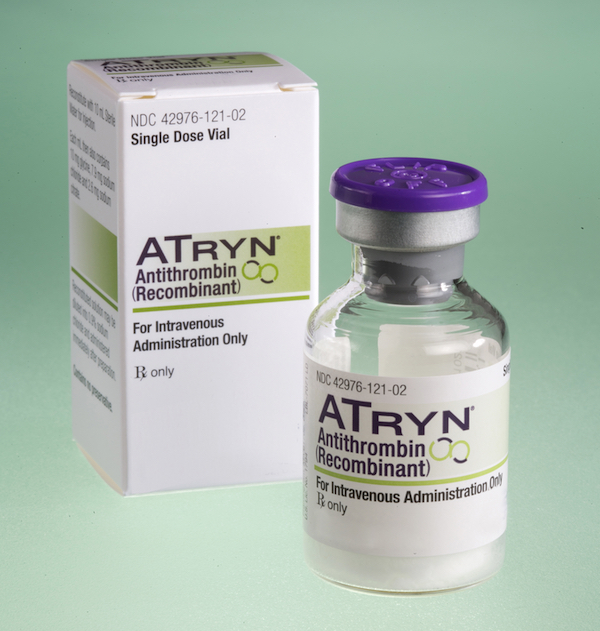 Transgenic goats produce milk with an anticoagulant that is then extracted for use in the drug ATryn. In the eyes of the government, goats can be pharmaceutical manufacturers, but they can’t also be the drug delivery system.
Transgenic goats produce milk with an anticoagulant that is then extracted for use in the drug ATryn. In the eyes of the government, goats can be pharmaceutical manufacturers, but they can’t also be the drug delivery system.Murray laughs, but it’s the tired laugh of someone who has spent most of his life pushing back against a reality he finds both absurd and beyond his control. And you might have to agree with his logic when you consider that a 2006 human trial in Peru showed that human lysozyme derived from transgenic rice was highly effective at treating childhood diarrhea. Or when you look at ATryn, a popular anticoagulant available on the market since 2009 that is produced from the milk of a herd of transgenic goats made with the same technology that Murray and Maga used to create Artemis. In the eyes of the government, goats can be pharmaceutical manufacturers, but they can’t also be the drug delivery system.
And that raises still another problem. Diarrhea claims the lives of very few Americans. It’s prevalent in places like sub-Saharan Africa, South America, and Southeast Asia places where upwards of 60 percent of the population are farmers, places where it would be far easier to raise a transgenic goat than to store, transport, and administer a drug isolated from one. So consumers in the First World are distanced from any direct advantages of products derived from genetic technologies. The opposition to them reflects not so much a presence of risks but an absence of benefits at home. Van Eenennaam laments that those in well-off countries don’t care about the 25,000 people who die from starvation every day. “I think it’s activist pressure on politicians that drives these decisions,” she says, “which is why we need applications that the general public understands.”
Spotigy (pronounced Spotty Guy) and Buri are 14-month-old male twins, equal parts playful and moody. Like all Holsteins, they are black and white with floppy ears and pink noses. But unlike the vast majority of their breed, they have never known the 1,000-degree-Fahrenheit heat of a dehorning iron an instrument dairy farmers use routinely on new calves to keep them from growing horns, which can be dangerous to farmers and other cattle. The soft whorls of hair at the top of Spotigy and Buri’s heads don’t hide scars because the twins never grew horns in the first place.
They owe their hornlessness to precision gene editing, a new technology that directs DNA-cutting enzymes to targeted locations in the genome. Bits of genetic code can be modified, removed, or replaced on demand. The procedure known as zinc finger nucleases, TALENs, or CRISPR-Cas9, depending on the technique used — makes it possible to change or disable a single gene without influencing the rest of the genome, so undesired effects are far less likely than with previous techniques. Scientists are calling it a revolution.
This is the technology that researchers at a startup called Recombinetics in St. Paul, Minnesota, used to make Spotigy and Buri. They subbed out the bit of DNA that makes dairy cattle have horns for the one that makes Angus beef cattle have none. Hornlessness occurs naturally in some breeds, but those animals, known as “polled,” tend to be inferior milk producers, and the trait hasn’t yet been successfully cultivated using conventional breeding methods. Then in 2012, animal geneticists identified the polled gene, and Scott Fahrenkrug saw an opportunity to pursue the quest for hornless Holsteins. He quit his tenured position at the University of Minnesota and became the full-time CEO at Recombinetics, which he had founded several years earlier.
Fahrenkrug was no genetic-engineering neophyte. He was one of the researchers rafting down the Truckee in the early 2000s, and that’s where he met Murray, Maga, and Van Eenennaam. It’s also why his gene-edited cows are all the way out here in California. Recombinetics doesn’t have a large-animal facility of its own, so when the bull calves turned six months old they were transported to Davis. With the help of a $434,000 food security grant from the USDA, Murray and his students will spend the next two years gathering data about the twins and monitoring their health.
Results are already coming in. In a paper published last month in the journal Nature Biotechnology, Fahrenkrug’s team reported perhaps their most compelling piece of evidence yet: There were no “off-target,” or unwanted effects to the rest of the twins’ genomes as a result of gene editing, one of the largest criticisms of the new technique. An accompanying editorial in the journal called for the FDA to not require pre-market approval for animals containing native and heirloom versions of a gene, even when introduced via gene editing (like Buri and Spotigy). Instead, the authors suggested that such livestock products should qualify for GRAS status, “given that DNA is generally regarded as safe to consume,” under current FDA guidelines.
Jaydee Hanson, a senior policy analyst for the consumer advocacy group Center for Food Safety, disagrees. “When you’re doing the first-of-a-kind product, how in heaven’s name do you say that this is generally regarded as safe when you’ve never seen it before?” Hanson, who before joining CFS served as the United Methodist Church’s staff director of genetics and bioethics and on the American Association for the Advancement of Science’s Science and Religion Advisory Committee, says that many scientists are asking the public to have a faith-based approach to gene editing, which is ironic. “At CFS, we haven’t said, ‘Thou shalt never genetically engineer something,’ we’re just saying it should be properly reviewed.” he says. “I want more than one generation of animals. I want to see what happens to the second and the third. That should be our standard.”
Van Eenennaam, who is overseeing outreach for the Davis-Recombinetics collaboration, says that’s what they’re working toward. When Buri and Spotigy are old enough to breed later this summer, their sperm will be harvested and used to create a new herd of hornless cattle to be studied. But even with great data, she recognizes that it will still be a tough sell. “I think what’s at the heart of it is how science and data works,” she says. “I could never say, ‘It is safe.’ I’ll never be able to say that. I can talk about relative risks, but not that. And activists use that uncertainty.”
For now, the public has yet to make up its mind about the Recombinetics cows, making Fahrenkrug and Van Eenennaam hopeful they can serve as a test case to show American consumers that gene editing can be harnessed to address issues of animal welfare, environmental impact, and human health. And if they’re right, Spotigy and Buri could be just the beginning.
The 2012 USDA grant for Fahrenkrug’s gene-editing efforts effectively lifted the regulatory dry spell of the previous decade. Van Eenennaam calls it a “game changer.”
Since then dozens of USDA grants have been awarded to agricultural researchers trying to improve livestock via genome editing. And in April the agency declared that it would not regulate a common white mushroom that has been modified with CRISPR to resist browning. The mushroom is one of about 30 GMOs to sidestep the regulatory system in the past few years — almost all plants. In each case, the agency’s Animal and Plant Health Inspection Service (APHIS) has said that the gene-edited crops are “not subject” to the same regulations as other GMO crops under the current framework, because they do not contain foreign DNA from plant pests such as viruses or bacteria. Such organisms were necessary for modifying plants in the 80s and 90s, but the new gene editing techniques don’t use them. The specificity of existing regulatory rhetoric has left a CRISPR-shaped loophole in the regulatory process, for now.
Animal researchers are seizing the opportunity to reimagine the genomes of an entire menagerie of creatures, not just for medical models and drug production. For the first time in years, scientists are urgently investing time and money in next-generation livestock. Projects underway include chickens that produce only female offspring. According to the animal rights group Mercy for Animals, more than 200 million day-old male chicks are now killed in grinders each year by the egg industry, which needs many more hens than roosters. Van Eenennaam is working on a line of bulls that would produce all-male herds of cattle to improve beef production efficiency. Also on the way are more hygienic honeybees, better-muscled sheep, and a host of animals that won’t need antibiotics because they’ll be born resistant to disease. This flurry of research represents a concerted movement by scientists to show the usefulness of the technology while there’s still time to influence regulators.
At the Roslin Institute in Edinburgh, Scotland, Bruce Whitelaw has changed three parts of one gene in domesticated pigs to make them more like wild pigs that are resistant to the devastating African swine fever. Though it’s not year clear whether they are resistant, those animals are already on the ground in Britain, thanks to investments from a commercial partner (The European Union is expected to rule on the regulatory status of gene editing within the year). Whitelaw says the agricultural industry’s recent interest in gene editing indicates a major shift in momentum the last three years. “What editing will do is increase, dramatically, the number of projects coming through,” he says. “The application numbers will be skyrocketing, and they [regulators] will need to do something about it.”
Recent events suggest that gene editing does seem to be forcing a long-overdue second look at GMO regulation. On July 2, 2015, the White House Office of Science and Technology Policy said that over the next year it would be revamping its protocols for genetically engineered crops and animals for the first time since 1992. In the last six months, the FDA, EPA, and USDA have all held public meetings to gather input on how gene editing is to be regulated. The USDA has also requested public comment on a proposed new framework for the regulation of GMO crops that would relax triggers for oversight. “In light of the experience we have gained over the past 28 years as well as continuing advances in biotechnology, we are beginning fresh stakeholder engagement aimed at exploring alternative policy approaches,” said a spokesman for APHIS.
Scientists hope these open discussions with the public and regulators will give them a chance to disentangle the issues of gene editing and shield it from the kind of bloc-force hostility that still plagues first-generation GMO products. While the discussion will undoubtedly be colored by the existing debate over the merits of genetic engineering writ large, the question on everyone’s mind is whether gene editing will lead to a regulatory loosening, to the benefit of languishing public-health projects like Maga and Murray’s goats. Or will the culture of fear created by GMOs of yesteryear continue to stifle innovation at a time when the technology is more precise and more affordable than it has ever been? With the chance for a regulatory do-over feeling closer than ever, scientists like Van Eenennaam, Maga, and Murray are starting to feel cautiously optimistic.
They don’t claim that biotechnology is a silver bullet. They understand that improving food security and public health without harming the environment will require the concerted use of many methods, from traditional breeding to organic farming. Genetic modification can’t hold back rising sea levels or fill aquifers drained by years of drought. But there are important contributions to be made with problems that have been unsolvable by other means, the researchers say — if only regulations would allow it.
“We believe the technology can do good for people,” says Maga. “I wouldn’t still be doing this if I didn’t really believe that.”
Back at Davis, Murray and Maga are now looking to expand the reach of their HLZ-goats, in senses both geographic and epidemiologic. Working with former students in Brazil, which has high diarrhea rates in some areas and a government interested in biotech solutions to the problems of poverty, they have cloned a second herd of transgenic goats. They’re still working through the red tape of starting human trials — something they couldn’t dream of doing in the U.S. — but for the first time it at least seems possible. “Elizabeth and I are stubborn and we refuse not to push forward with this,” says Murray. “[We] refuse to feel that this technology can afford to sit on the shelf.”
Maga is also finding ways to use her years of work to pivot toward a line of research with higher potential to succeed at home. She and a graduate student are developing a pig model for Inflammatory Bowel Disease. Two litters of gene-edited piglets were born in April and will soon be fed lysozyme-enriched goat milk to see if it can also ameliorate the symptoms of IBD. Maga’s colleagues in the medical school are eagerly awaiting the results, and she’s cautiously optimistic that the treatment could find traction because it is a more relevant disease than childhood diarrhea to a wealthy, developed nation such as our own.
And in the last few months, Maga says, the Gates Foundation has begun to express some interest in working with her on her original project. “We’re just trying to do something that can help people,” she says.
Megan Molteni is a freelance science writer, producer and researcher, specializing in biology, technology and the environment. Her work has appeared in Popular Science, Discover Magazine, Aeon, The Riveter, and Conservation Magazine.
This article has been updated to clarify the federal evaluation status of recombinant human lysozyme derived from transgenic rice. While the Food and Drug Administration twice received notices from a Colorado company, Ventria Bioscience, declaring the presumed safety of its rice-derived lysozyme for use as an additive in a variety of foods — a preamble to FDA evaluation of such safety claims — the company later withdrew both notices, and the agency discontinued its evaluations. To date, the FDA has not sanctioned use of this product in food.











Comments are automatically closed one year after article publication. Archived comments are below.
I agree with you
The GMO debate in the world, especially Western Europe and increasingly the United States is symptomatic of many discussions. Greenpeace was one of the original fighters of GMO foods. Greenpeace develops a marketing plan to raise money. If people will donate, then more money is put in the action. Data does not matter, money does. Greenpeace sells fear. Greenpeace was part of the development of Golden Rice, which would economically improve the nutrition of about 250 million people mostly in South Asian and Africa. Greenpeace was part of the discussion, including development and only contested the project when Golden Rice started the regulation process. An estimated 8 million children die each year from Vitamin A deficiency. Greenpeace response, the people who subsidence farm, should buy Vitamin A from 1st world countries or more likely China. Since the subsidence farming communities have not money, they have diseases from lack of Vitamin A.
GM crops exist for three reasons: 1. GM crops are profitable for the producer, for the farmer (reduces total costs) and the consumer (lower prices) 2. GM crops reduce total costs, related to point 1 but the driver: less chemicals (pesticides, herbicides), fuel (application of chemicals) and increased yield. 3 GM crops improve nutrition, the first crops increased calories (you can argue the importance of this given world wide obesity) but the new GM crops are targeting other nutritional needs: Vitamin deficiency, improved fat profile (high oleic soybean), more stable fruits and vegetables.
The next step in GM products is developing new cures for diseases as this article reviews. GM will (research started) cure genetic diseases by changing a person’s chromosomes. New treatments for cancer, HIV and other diseases are starting to come to market. The issue for the Western World is do we listen to he fear of people who will not listen to the science or does the Western World develop safe methods to use GM for food and diseases. China conducts GM research and is now leading the world on the use of GM technology on humans. The issue is not will GM drugs and modifications happen but who will control the GM technology?
@Zoe Lafantaisie: Why the surprise? If it were not for “eugenics” then the current modern day versions of cattle, pigs, sheep and chickens would not exist in the animal kingdom. In the wider organic world modern vegetables that you eat every day – hopefully! – would also not exist. This research is basically a more modern and efficient way to obtain better selective breeding of traits, characteristics and propertied of naturally occurring qualities just as the first farmers did with the range of natural products that they eventually realised could be manipulated to the wider benefit of their species.
Surprised to see UnDark promoting eugenics – too bad – I will be unsubscribing – you CANNOT improve on nature – just stop this insanity. It is time to nurture the earth, worth WITH animals, not on them. Scientists have lost their minds and I guess so has this tool of the GMO industry.
“In the early 2000s the USDA stopped accepting grant proposals for transgenic animals.”
It is not exactly true that USDA stopped funding transgenic animal research in 2000. I directed a program at USDA-CSREES from 2004 to 2008 and funded Jim Murray’s research on transgenic swine during that time period. There was never any deliberate effort to not fund such research – it was up to the panel considering all types of transgenic research to decide which proposals were funded, and therefore which were not. Money is always limited as to what gets funded and which proposals do not. One is always up against a variety of proposals with different perspectives and areas of research – USDA’s funding is minuscule compared to NSF and NIH.
Typo “evidencse”
Thanks!
I thoroughly understand the frustrations of Professors Maga and Murray having had an hand in the development of the Enviropig by Dr. Cecil Forsberg at the University of Guelph, Canada, which enables the secretion of the Phytase enzyme from the pig salivary gland to degrade Phosphorus and convert it into a less polluting form. After 12 years of hard work and several thousand pages of regulatory material submitted to USFDA, there was no commercial interest for a GMO pig, even from a pork powerhouse that is China. It was a sober realization to all of us who were involved in this project, that commercial path to success is more important than sheer technical prowess and the brilliance of our ideas to ameliorate major issues associated with livestock. Additionally, I was witness to the now defunct spider silk program from goat milk (another path breaking GMO project initiated at the Montreal based Nexia Biotechnologies where they could not develop the technology enough to spin silk thread. However, that idea has been revived and now close to commercialization by using bacteria to produce spider silk, one from Japan based Spiber and by Bolt threads in Silicon Valley.
The lysozyme producing GMO goat is too important to let it languish in limbo. If any country has the ambition and moved quickly to develop GMO animals, it is China. They seem to possess the drive, the resources and the possibility of making profits from new biotech platforms, with speedy regulatory approvals. Personally, I know that a scientist who worked on the goat spider silk project in Montreal has moved to China and started a biotech company to produce human enzymes for unique diseases from pathogen free genetically modified rabbits.
It is my hope that both the lactoferrin producing cow project and the lysozyme producing goat project be out licensed to a chinese group or developed jointly as a collaboration between UC Davis angels and a chinese partner. The path to regulatory approval is easier and the product can be produced in large quantities in an industrial farm scale animal project.
I have hemophilia A, or Factor XIII hemophilia. I was told nearly 20 years ago the next big thing treatment was going to be clotting factor produced by transformed milk cows or goats. This process would presumably lead to less expensive factor than that used now, in which transformed Chinese hamster ovary cells are conned into producing the factor. This factor is mind-bogglingly expensive. In 1995 I had surgery on a damaged elbow; the factor necessary for the surgery and recovery ran to nearly half a million dollars. 20 years later, the hemophiliacs of the world are still waiting.